Water Vapor Chart
Water Vapor Chart - These maps show satellite measurements of water vapor for a given day, or over a span of days. Each monthly map shows where water vapor is above or below the average for that month over the period of 1979 to 2015. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. Note that when water vapor pressure equals atmospheric pressure, then the water molecules are free to jump into the gas state. Web water vapor pressure chart. To establish proper comfort conditions within a building space, the designer must consider the air temperature and the amount of water vapor in the air. The maps are based on data collected by the moderate resolution imaging spectroradiometer ( modis) sensor on nasa's aqua satellite. Web the lowest amounts of water vapor (0 centimeters) appear in white, and the highest amounts (6 centimeters) appear in dark blue. Web the heat energy (enthalpy of evaporation) needed at 7 bar g to vaporize the water to steam is actually less than required at atmospheric pressure. It is one state of water within the hydrosphere. Vapor pressure of h 2 o at various temperatures (celsius) modified from nebergall et. Using this 1st calculator, you insert temperature in °f, and get the vapor pressure of water in terms of kpa, psi, mmhg, bar, atm, torr. Web water vapor pressure chart. Vapor pressure is directly proportional to temperature). Web so scientists monitor water vapor because it influences. Each monthly map shows where water vapor is above or below the average for that month over the period of 1979 to 2015. Vapor pressure is directly proportional to temperature). Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Web so scientists monitor water vapor because it influences earth's weather. To establish proper comfort conditions within a building space, the designer must consider the air temperature and the amount of water vapor in the air. Note that when water vapor pressure equals atmospheric pressure, then the water molecules are free to jump into the gas state. Web the heat energy (enthalpy of evaporation) needed at 7 bar g to vaporize. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number, thermal diffusivity, entropy and enthalpy). Web generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The ineractive map makes it easy to navitgate around the globe. Web the heat energy. The saturation vapor pressure is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. Web water vapor, water vapour or aqueous vapor is the gaseous phase of water. Web generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This chart shows that this trend is true for various substances. We look at the 68°f example specifically. 99.974 °c = 211.953 °f. Vapor pressure of water from 0 °c to 100 °c. To establish proper comfort conditions within a building space, the designer must consider the air temperature and the amount of water vapor in the air. Web the vapor pressure of water is the pressure exerted by molecules of. Web explore a comprehensive table of water vapor pressure at different temperature values presented in both si (kpa) and us customary (psi) units. Using this 1st calculator, you insert temperature in °f, and get the vapor pressure of water in terms of kpa, psi, mmhg, bar, atm, torr. Vapor pressure is directly proportional to temperature). Water vapor can be produced. Areas of missing data appear in shades of gray. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. Web the heat energy (enthalpy of evaporation) needed at 7 bar g to vaporize the water to steam is actually less than required at atmospheric pressure. This chart shows. Web water vapor pressure chart. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. These maps show satellite measurements of water vapor for a given day, or over a span of days. Web the heat energy (enthalpy of evaporation) needed at 7 bar g to vaporize the. The specific enthalpy of vaporization decreases with steam pressure. This chart shows that this trend is true for various substances with differing chemical properties. It is one state of water within the hydrosphere. Areas of missing data appear in shades of gray. Note that when water vapor pressure equals atmospheric pressure, then the water molecules are free to jump into. Vapor pressure of h 2 o at various temperatures (celsius) modified from nebergall et. This chart shows that this trend is true for various substances with differing chemical properties. Note that when water vapor pressure equals atmospheric pressure, then the water molecules are free to jump into the gas state. Vapor pressure of water from 0 °c to 100 °c. The maps are based on data collected by the moderate resolution imaging spectroradiometer ( modis) sensor on nasa's aqua satellite. We look at the 68°f example specifically. Web see the latest united states water vapor weather satellite map. The saturation vapor pressure is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. Areas of missing data appear in shades of gray. It is one state of water within the hydrosphere. Web thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting, latent heat of evaporation, critical temperature and more. The specific enthalpy of vaporization decreases with steam pressure. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. Web the vapor pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as air). Web generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity, thermal conductivity, specific heat, prandtl number, thermal diffusivity, entropy and enthalpy).
Table Of Vapor Pressure Of Water A Guide To Understanding Its
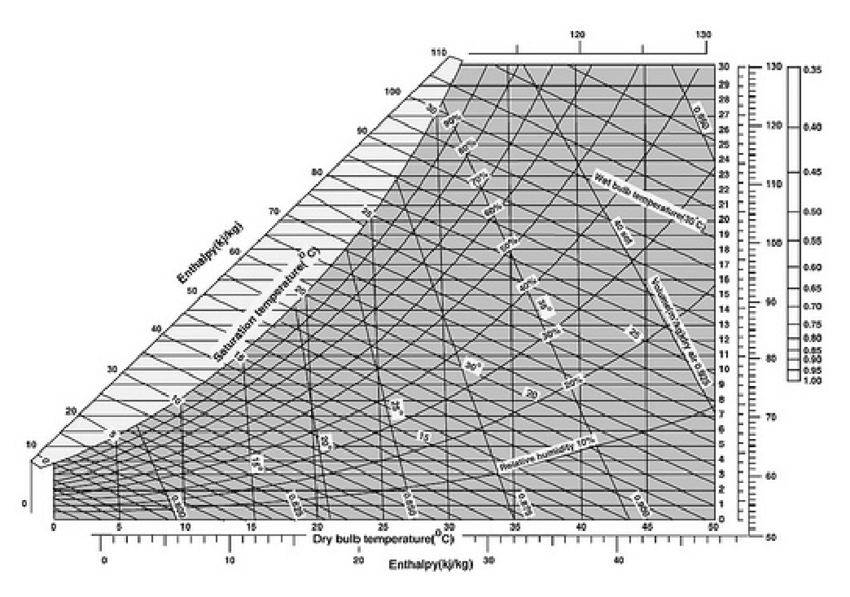
Water Vapor Chart A Visual Reference of Charts Chart Master

vapor pressure of water table
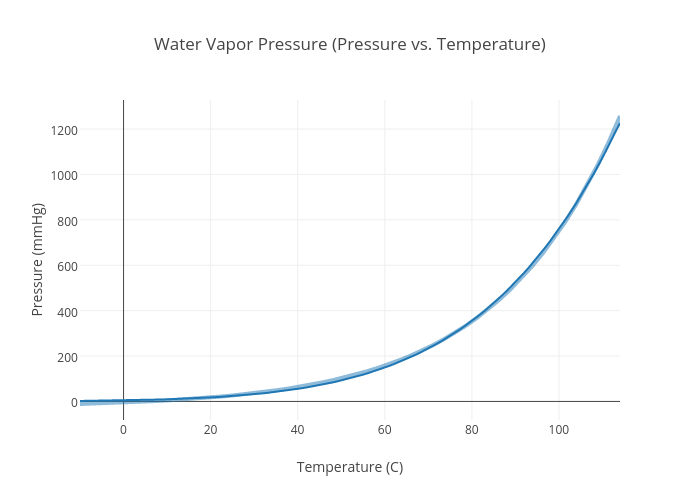
Water Vapor Pressure Temperature Chart
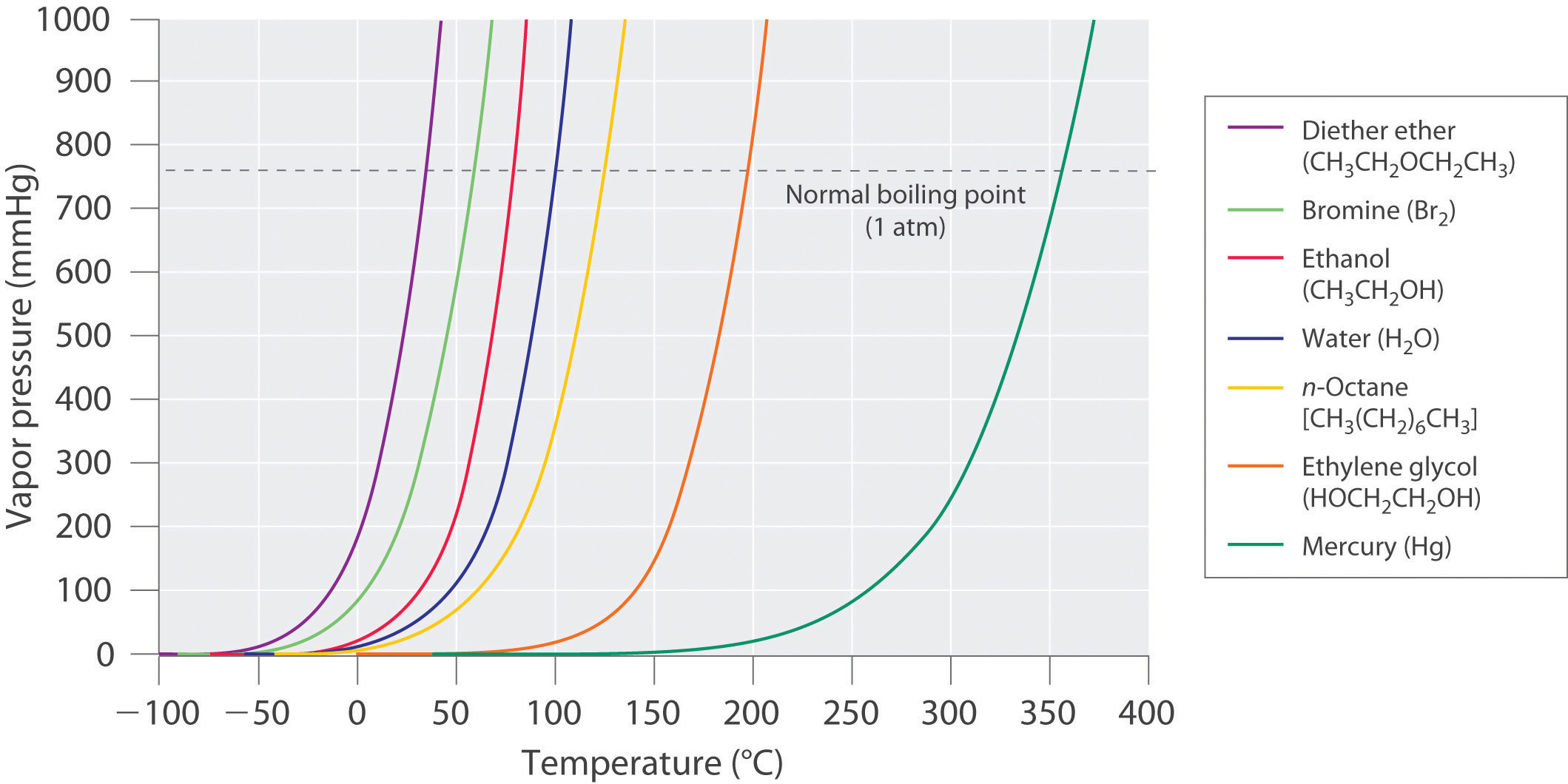
Chapter 11.4 Vapor Pressure Chemistry LibreTexts
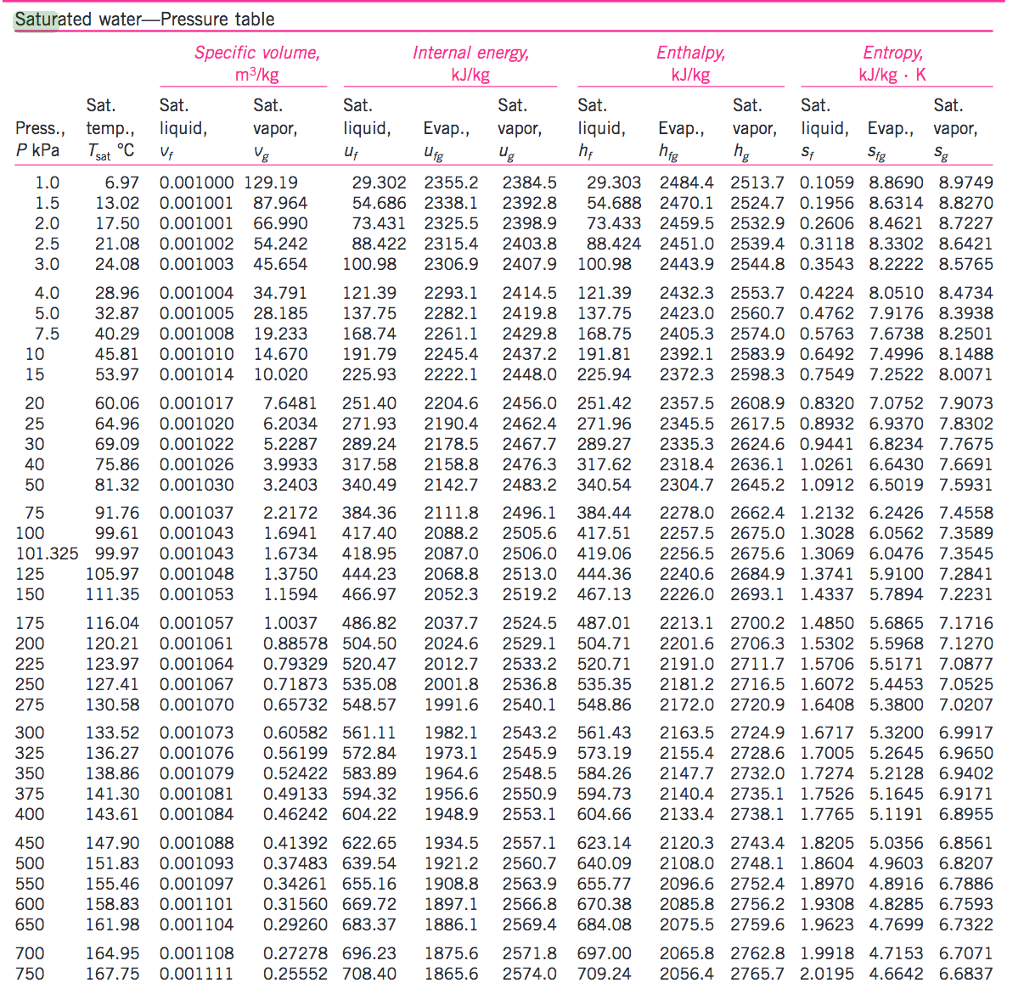
Vapor Pressure Chart For Water

Water Vapour Pressure Chart Bar
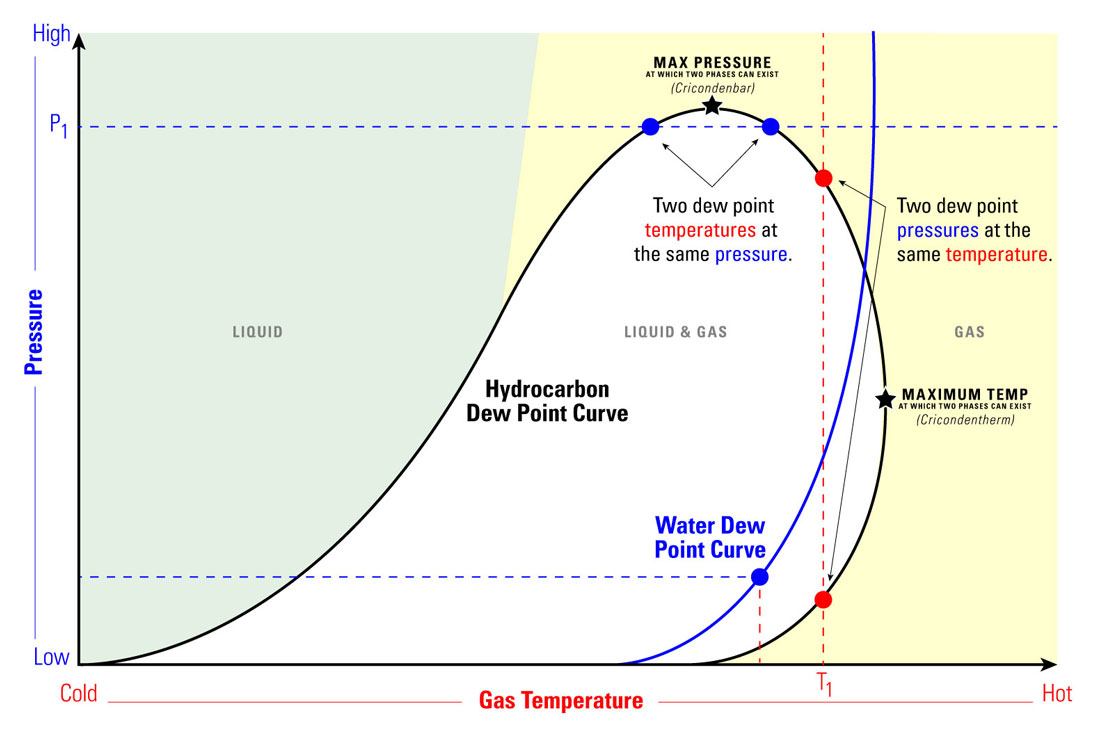
What's the Difference between Hydrocarbon Dew Point and Water Vapor Dew

Conservation physics Fundamental microclimate concepts

Water Vapor Chart
Water Vapor Can Be Produced From The Evaporation Or Boiling Of Liquid Water Or From The Sublimation Of Ice.
Web The Lowest Amounts Of Water Vapor (0 Centimeters) Appear In White, And The Highest Amounts (6 Centimeters) Appear In Dark Blue.
Web The Heat Energy (Enthalpy Of Evaporation) Needed At 7 Bar G To Vaporize The Water To Steam Is Actually Less Than Required At Atmospheric Pressure.
The Pressure Up Cancels The Pressure Down And Boiling Begins.
Related Post: