Chart Polyatomic Ions
Chart Polyatomic Ions - 2 lists the ion names and ion formulas of the most common polyatomic ions. Examples include sulfate, so−2 4, nitrate, n o− 3, and phosphate, p o−3 4. Web table of polyatomic ions. Web other ions consist of a group of atoms with a net charge. Web this polyatomic ions list contains many common polyatomic ions grouped by charge. Polyatomic ions have characteristic formulas, names, and. Web the table below lists a number of polyatomic ions by name and by formula. Because these ions contain more than one atom, they are called polyatomic ions. Isn't it proper to write c2h3o2? Web these polyatomic ions are extremely common in chemistry and thus it is important to be able to both recognize and name them. They have a giant lattice structure with strong ionic bonds. While there are many such ions in the world, you are responsible for knowing the ions listed in the following tables. It is worth committing the polyatomic ions to memory, including their molecular formulas and ionic charge. Because these ions contain more than one atom, they are called polyatomic ions.. Web a polyatomic ion (also known as a molecular ion) is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. 2 lists the ion names and ion formulas of the most common polyatomic ions. Because. 1 lists the ion names and ion formulas of the most common polyatomic ions. Web this polyatomic ions list contains many common polyatomic ions grouped by charge. Web being familiar with the names, charges, and formulas of the most common polyatomic ions will be helpful for recognizing ionic compounds and predicting their reactivity. Web table of polyatomic ions. It is. Because these ions contain more than one atom, they are called polyatomic ions. Web common polyatomic ions ; This is the structure of. Web other ions consist of a group of atoms with a net charge. Web a polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. It has one nitrogen atom and three oxygen atoms and an overall 1− charge. This is the structure of. Web common polyatomic ions ; Since these ions are composed of multiple atoms covalently bonded together, they are called polyatomic ions. Web some ions consist of groups of atoms bonded together and have an overall electric charge. Reviewing the common polyatomic ions, and explaining common suffixes and prefixes to help remember the formulas. While there are many such ions in the world, you are responsible for knowing the ions listed in the following tables. Let's explore some of the most common polyatomic ions and learn how to write chemical formulas for compounds containing these ions. They have. For example, no−3 no 3 − is the nitrate ion; Polyatomic ion charge = +1. Web a polyatomic ion (also known as a molecular ion) is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero.. Since these ions are composed of multiple atoms covalently bonded together, they are called polyatomic ions. Note that the vast majority of the ions listed are anions—there are very few polyatomic cations. There are a number of patterns that can greatly reduce the amount of memorizing that one must do. It has one nitrogen atom and three oxygen atoms and. Cr cr o o o. This is the structure of. Web a polyatomic ion (also known as a molecular ion) is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. Web poly atomic ions are. Reviewing the common polyatomic ions, and explaining common suffixes and prefixes to help remember the formulas. Web this is a list of some of the most common polyatomic ions. Web a polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. The heading for each column indicates the charge on the polyatomic ions in that. 1 lists the ion names and ion formulas of the most common polyatomic ions. 2 lists the ion names and ion formulas of the most common polyatomic ions. Because these ions contain more than one atom, they are called polyatomic ions. Reviewing the common polyatomic ions, and explaining common suffixes and prefixes to help remember the formulas. Here's a guide to some of the most common examples! Web polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. Since these ions are composed of multiple atoms covalently bonded together, they are called polyatomic ions. For example, no−3 no 3 − is the nitrate ion; Web polyatomic ions are molecular ions composed of two or more atoms bonded by covalent bonds and acting as a single unit, but unlike molecules, they have a net charge on them. It has one nitrogen atom and three oxygen atoms and an overall −1 charge. The following table lists some of the common polyatomic ions. Web other ions consist of a group of atoms with a net charge. Polyatomic ion charge = +1. Web common polyatomic ions ; It has one nitrogen atom and three oxygen atoms and an overall 1− charge. Cr cr o o o.
Polyatomic Ion Charts Find Word Templates
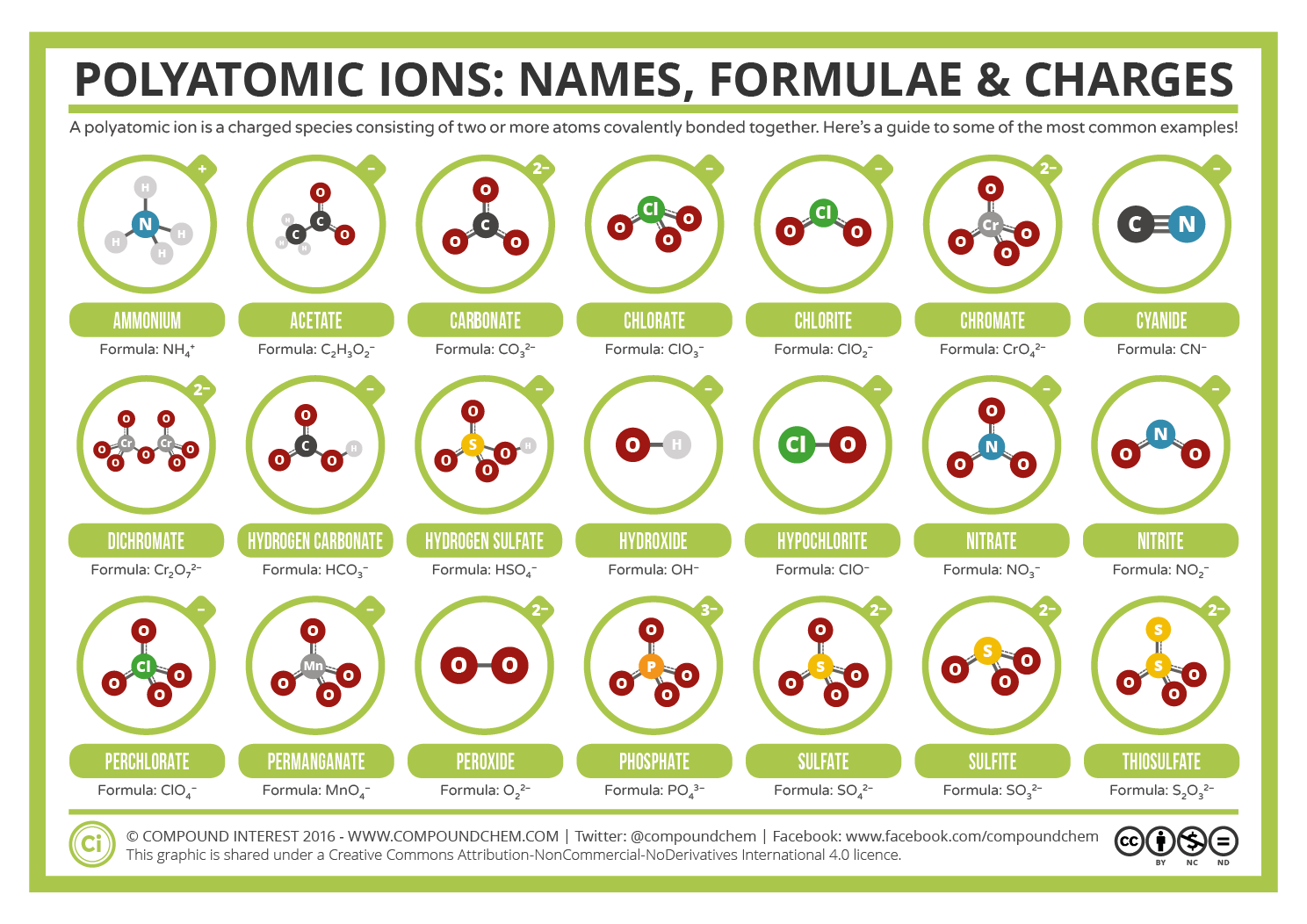
Common Polyatomic Ions Names, Formulae, and Charges Compound Interest
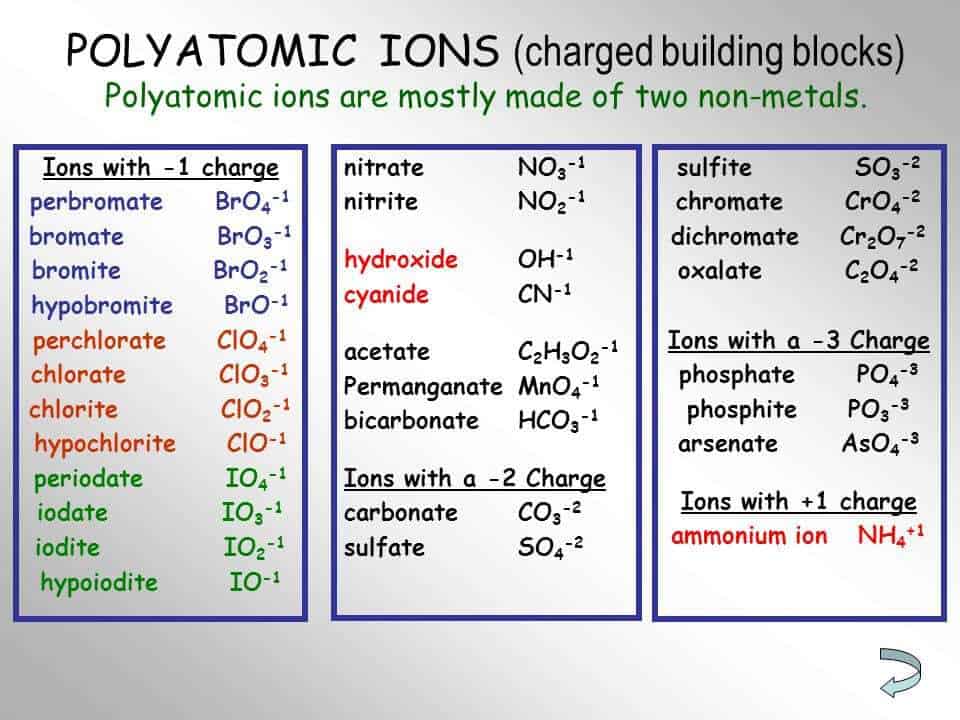
Polyatomic Ion Charts Find Word Templates
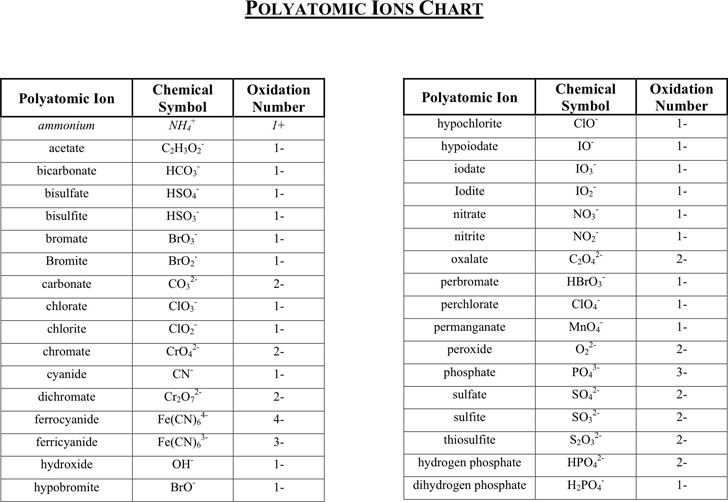
Free Polyatomic Ions Chart PDF 76KB 1 Page(s)
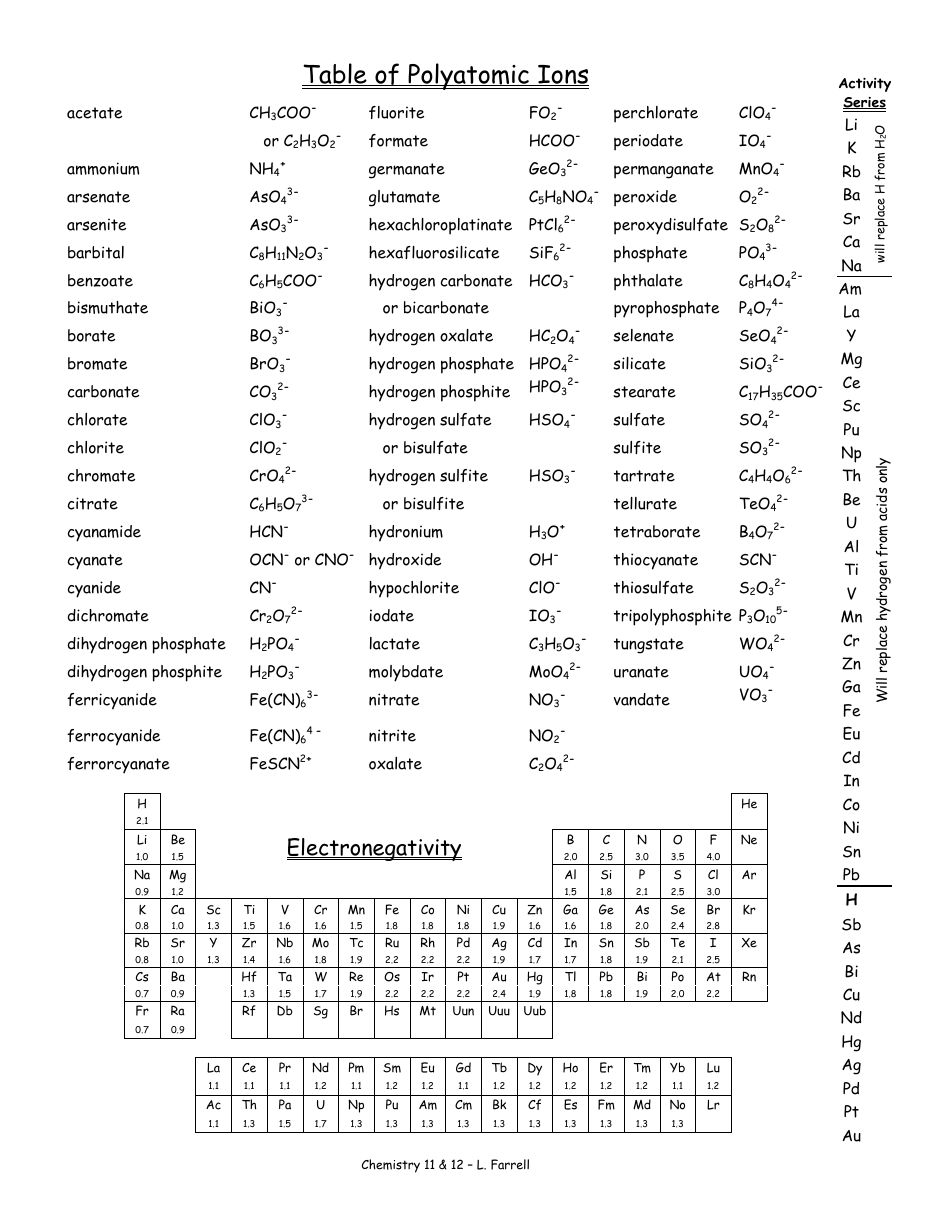
Polyatomic Ions Chart Download Printable PDF Templateroller
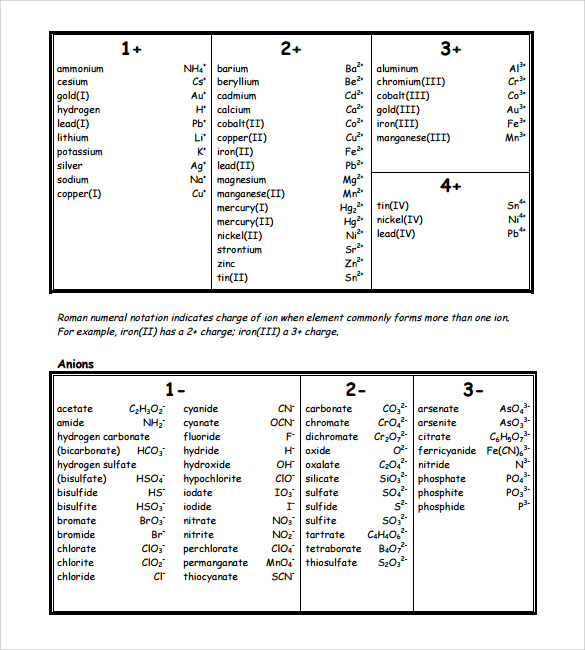
Chart Of Polyatomic Ions

Polyatomic Ions Chart 15 Free Templates in PDF, Word, Excel Download
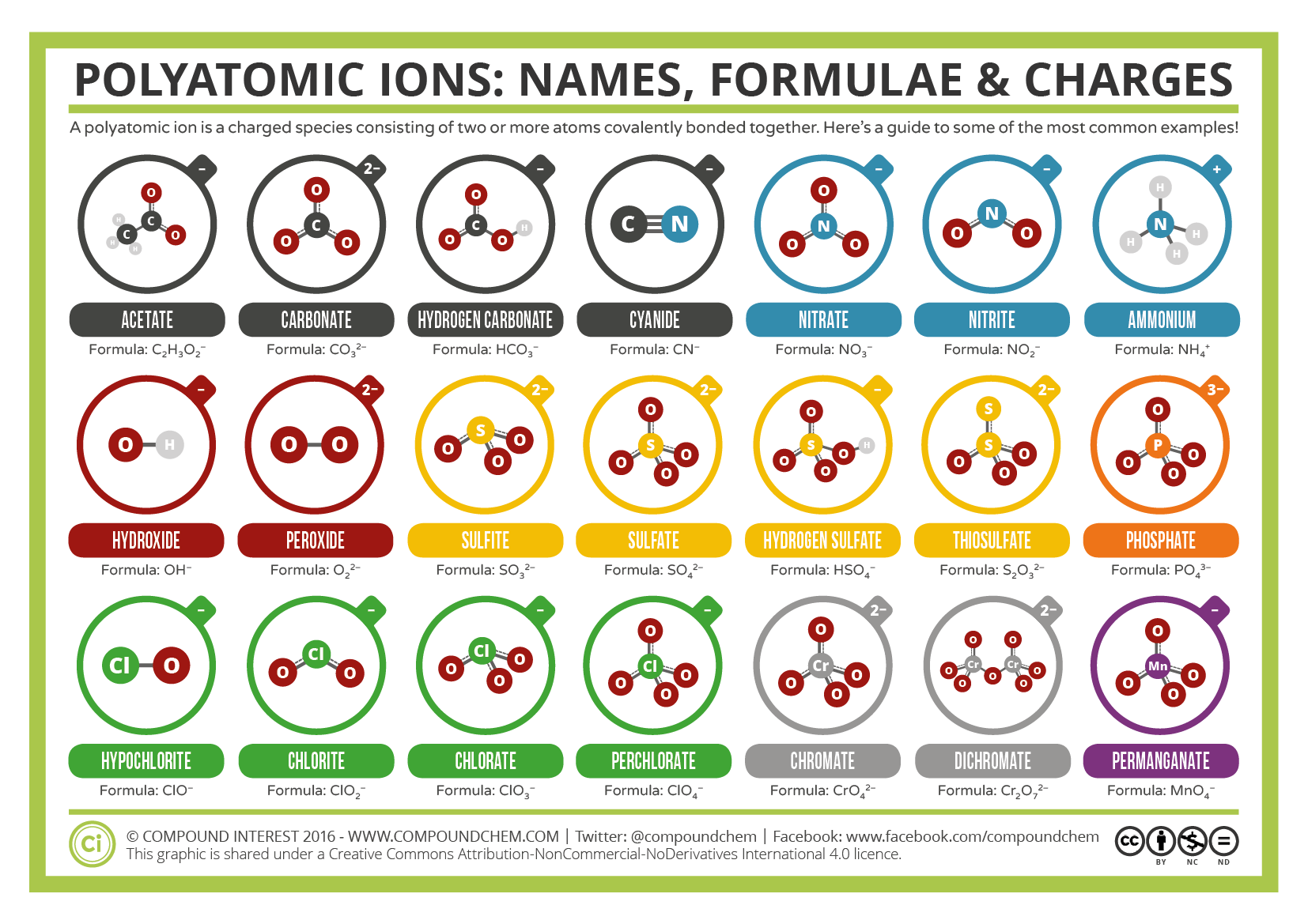
Common Polyatomic Ions Names, Formulae, and Charges Compound Interest
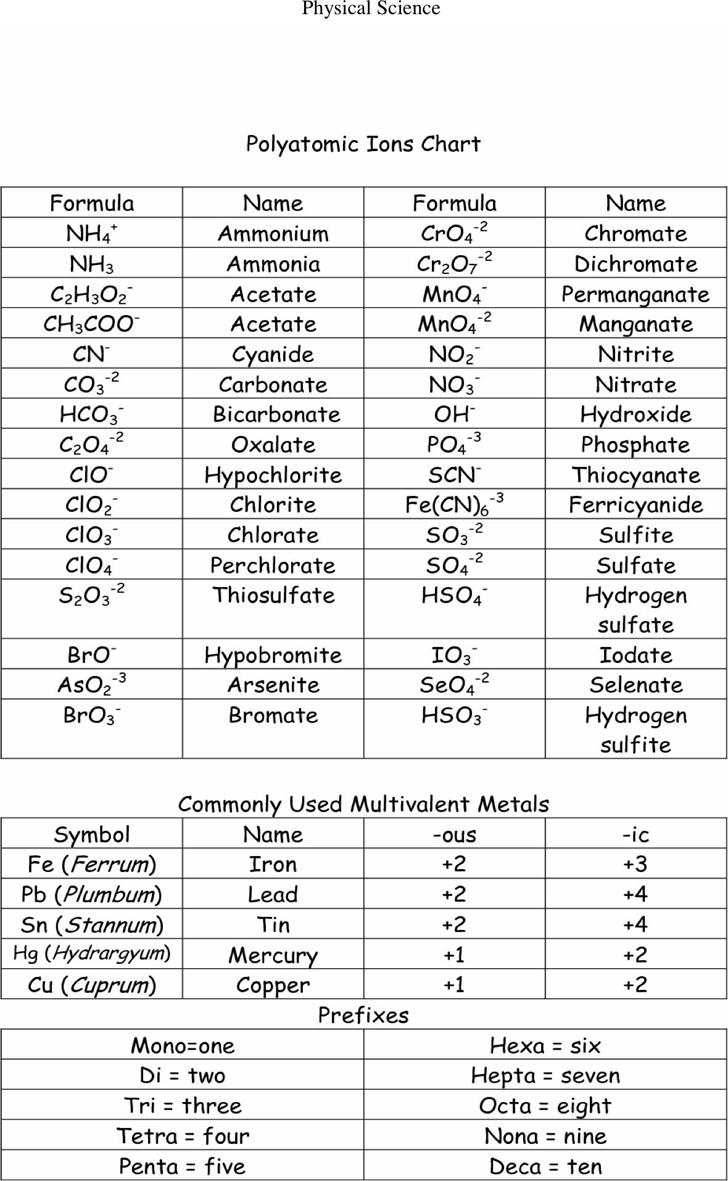
ions cl
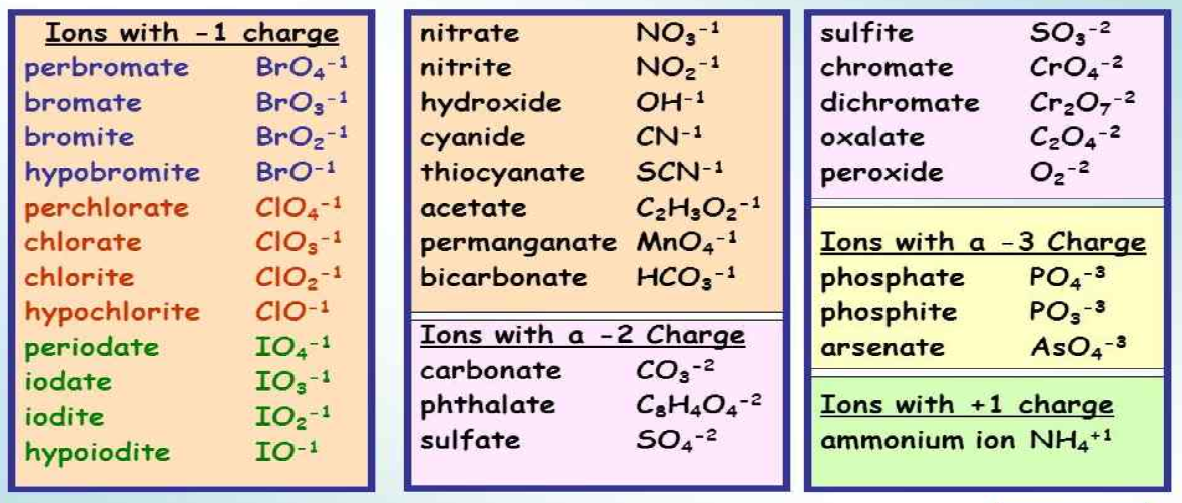
Polyatomic Ions Naming and Formulas Study Guide Inspirit
To Learn More About The List Of Polyatomic Ions, Monatomic Ions, Name, Charge And Faqs, Visit Byju’s
Web A Polyatomic Ion (Also Known As A Molecular Ion) Is A Covalent Bonded Set Of Two Or More Atoms, Or Of A Metal Complex, That Can Be Considered To Behave As A Single Unit And That Has A Net Charge That Is Not Zero.
The Heading For Each Column Indicates The Charge On The Polyatomic Ions In That Group.
Know Your Sulfates From Your Sulfites, And Your Chlorates From Your Perchlorates?
Related Post: