Dielectric Corrosion Chart
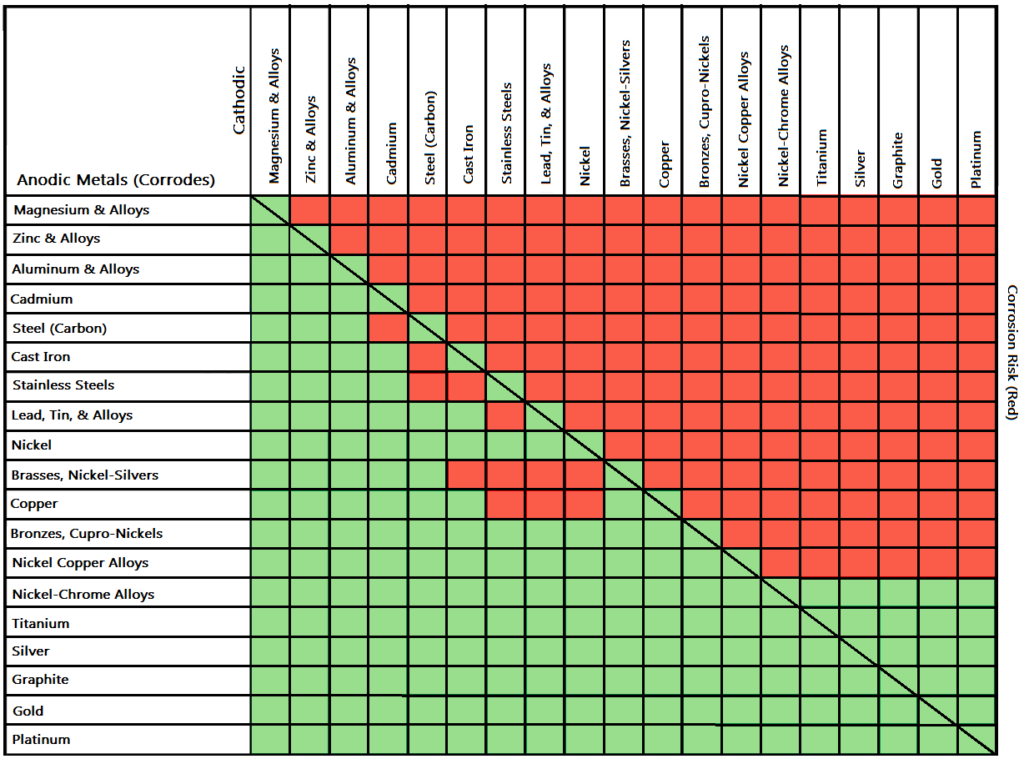
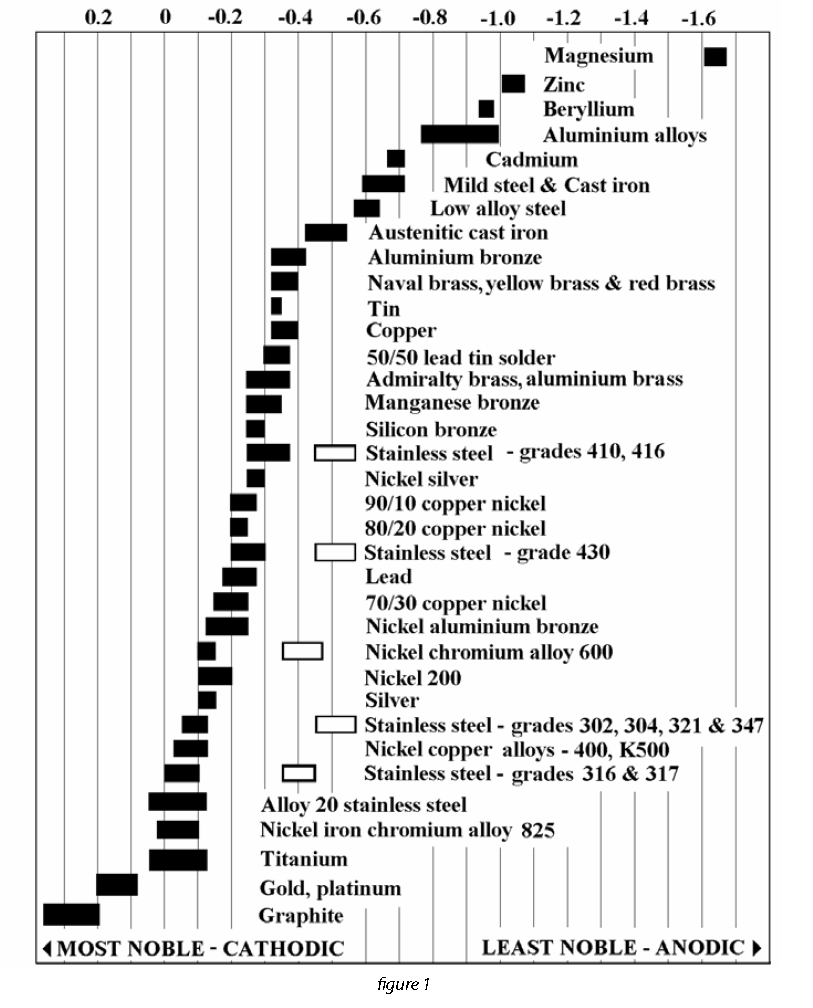
Dielectric Corrosion Chart - Web galvanic corrosion is an electrochemical process in which one metal corrodes preferentially to another when both metals are in electrical contact, in the presence of an electrolyte. First there must be two electrochemically dissimilar metals present. Such tables are of significant value in drawing the attention of designers to the dangers of unintended galvanic corrosion. The table, however, must be interpreted not only Web dielectric unions are designed to prevent galvanic corrosion, which can occur when dissimilar metals come into contact with each other. See the chart with anodic, cathodic, and neutral metals, and learn more about the factors affecting galvanic corrosion. There are three conditions that must exist for galvanic corrosion to occur. Web corrosion, with the use of charts depicting the galvanic (or electromotive force) series in different environments. Web this slide includes a chart of galvanic corrosion potential between common construction metals. Types of galvanic corrosion in different metals and their alloys. Web corrosion, with the use of charts depicting the galvanic (or electromotive force) series in different environments. Web this slide includes a chart of galvanic corrosion potential between common construction metals. Types of galvanic corrosion in different metals and their alloys. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water).. The galvanic series indicates which dissimilar metal will tend to corrode (anode) and which dissimilar metal will tend to support the reduction reaction (cathode), when the requirements for galvanic corrosion are met. They help to create a barrier between the two metals, which. The chart below shows how different plating materials react to one another with regard to their galvanic. When dissimilar metals are used together in the presence of an electrolyte, separate them with a dielectric material such as insulation, paint or similar surface coating. First there must be two electrochemically dissimilar metals present. What are the causes of galvanic corrosion? Web find out how different metals will corrode when placed together in an assembly based on their galvanic. Web dielectric unions are designed to prevent galvanic corrosion, which can occur when dissimilar metals come into contact with each other. The below galvanic corrion chart or anodic index table shows anodic index for different materials. A typical rule of thumb is that voltage differences of 0.2 volts or more suggest a galvanic corrosion risk. Learn how to identify, avoid,. First there must be two electrochemically dissimilar metals present. The table, however, must be interpreted not only Such tables are of significant value in drawing the attention of designers to the dangers of unintended galvanic corrosion. Types of galvanic corrosion in different metals and their alloys. Learn how to identify, avoid, and prevent galvanic corrosion with a chart of metal. Web galvanic corrosion is the damage of metal due to an electrochemical reaction between dissimilar metals in contact with an electrolyte. Web find out how different metals will corrode when placed together in an assembly based on their galvanic corrosion potential. What are the causes of galvanic corrosion? A typical rule of thumb is that voltage differences of 0.2 volts. In a structure, this corrosion galvanic corrosion when different metals come into contact with each other, one is likely to corrode faster if they get wet, but there are ways to minimise or even prevent corrosion. These products have safely been used for many years in those environments. Web the galvanic series chart below shows metals and their electrochemical voltage. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. They help to create a barrier between the two metals, which. Web galvanic corrosion is an electrochemical process in which one metal corrodes preferentially to another when both metals are in electrical contact, in the. Web this slide includes a chart of galvanic corrosion potential between common construction metals. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). Web dielectric unions are designed to prevent galvanic corrosion, which can occur when dissimilar metals come into contact with each other. Web what is the galvanic series?. Stainless steel (active) + aluminum Passive) under conditions which provide sufficient oxygen to the surface. These products have safely been used for many years in those environments. Figure 1 shows a galvanic table which lists metals from the least active (noble) to the most active. As a rule of thumb, if the wetted area of the corroding metal is 10. Web this slide includes a chart of galvanic corrosion potential between common construction metals. To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. Basics of aqueous corrosion four conditions must be present for corrosion to occur in aqueous solutions: Web corrosion will cause a failure, nor does it mean that dielectric fittings must be used at transitions between materials. Web dielectric unions are designed to prevent galvanic corrosion, which can occur when dissimilar metals come into contact with each other. Web corrosion, with the use of charts depicting the galvanic (or electromotive force) series in different environments. Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). Passive) under conditions which provide sufficient oxygen to the surface. As a rule of thumb, if the wetted area of the corroding metal is 10 times the wetted area of the noble metal, then galvanic A typical rule of thumb is that voltage differences of 0.2 volts or more suggest a galvanic corrosion risk. Web what is the galvanic series? Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Below is a galvanic reaction chart for dissimilar metals. The chart below shows how different plating materials react to one another with regard to their galvanic potential. There are three conditions that must exist for galvanic corrosion to occur.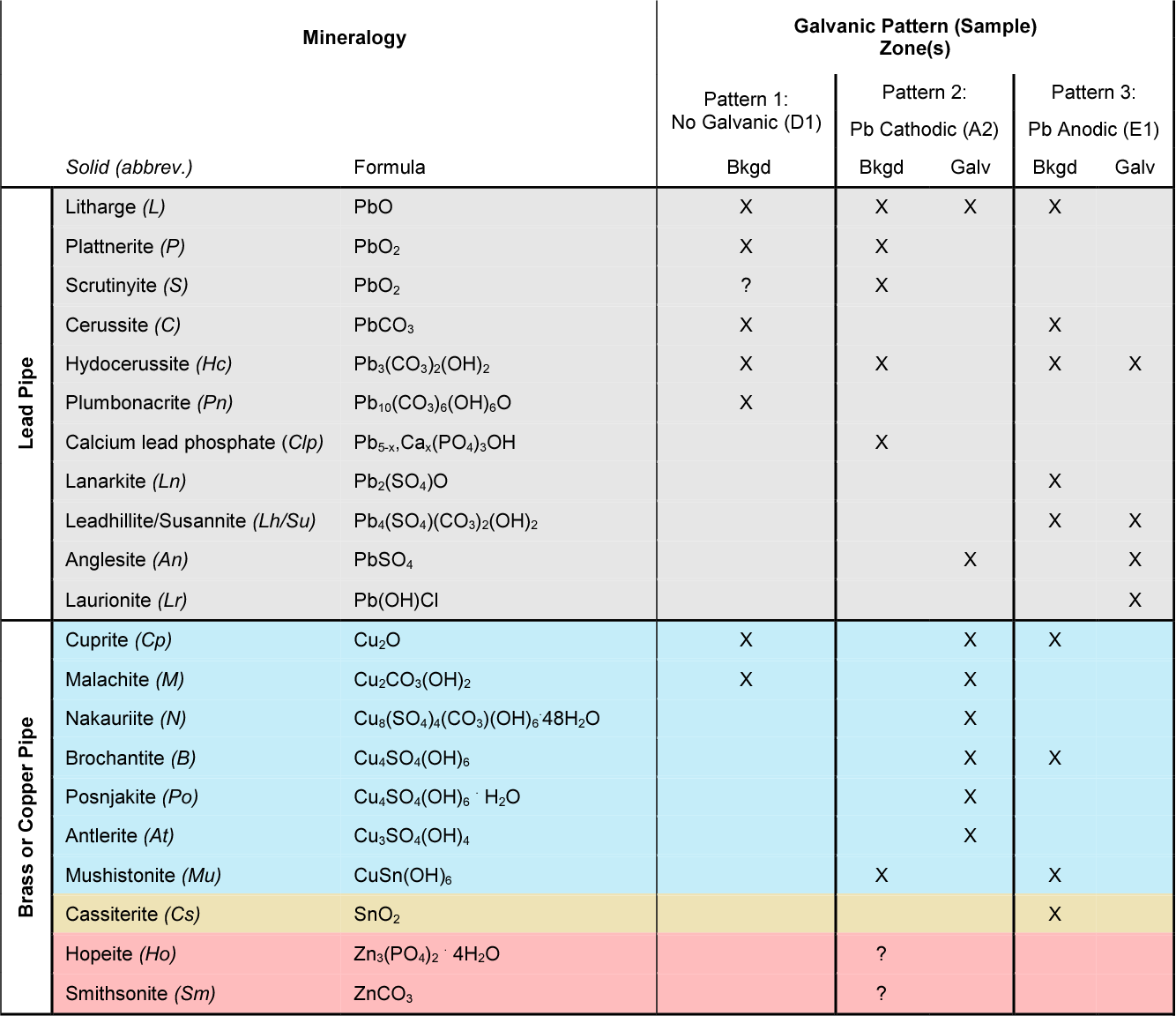
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master

Dielectric Chart
Architectural Metals Archives Monarch Metal
Dielectric Chart
The 5 W’s of Isolation Gaskets Triangle Fluid Controls Ltd.
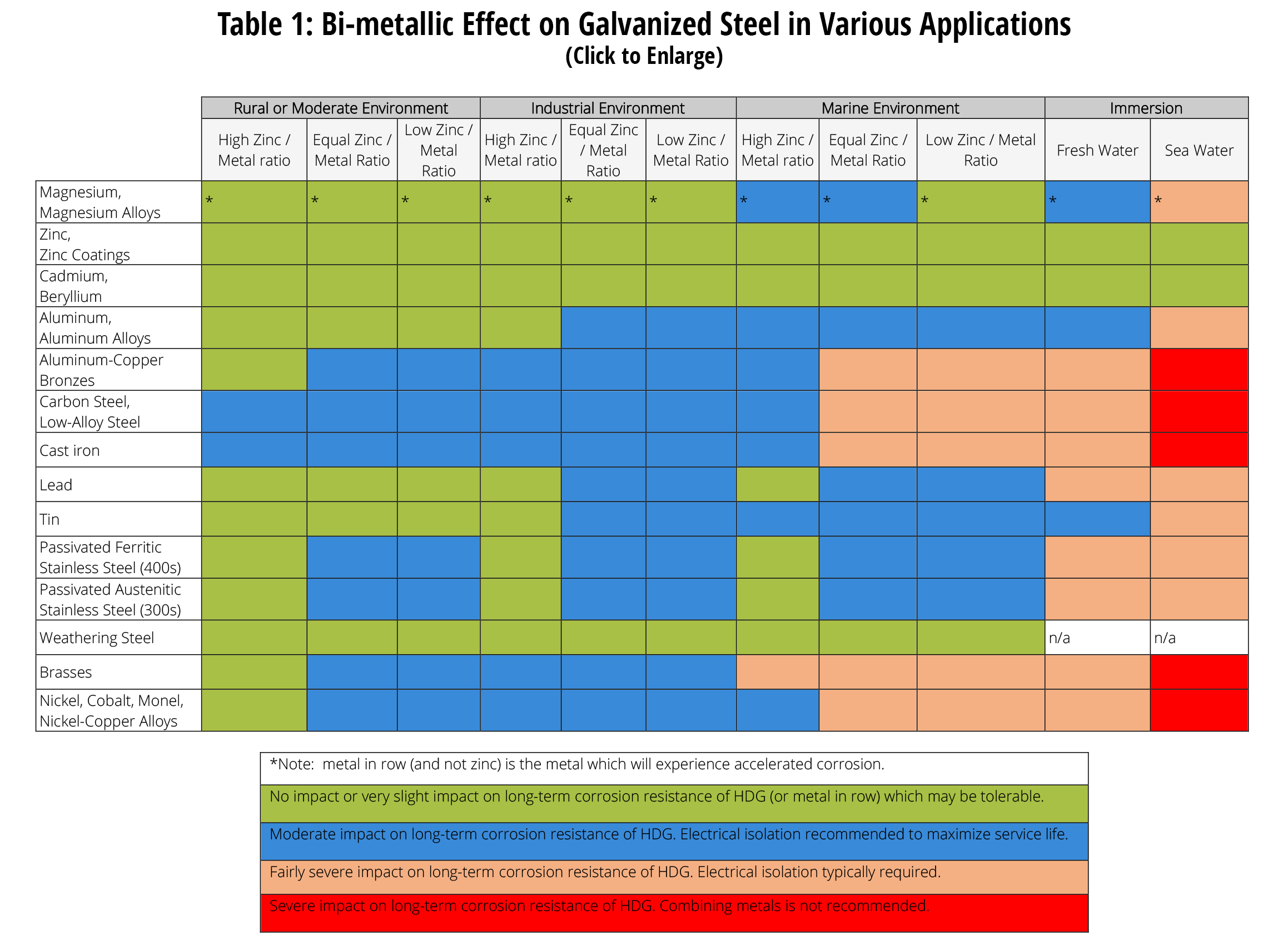
Dissimilar Metal Corrosion with… American Galvanizers Association

Corrosion charts Graphite Technology
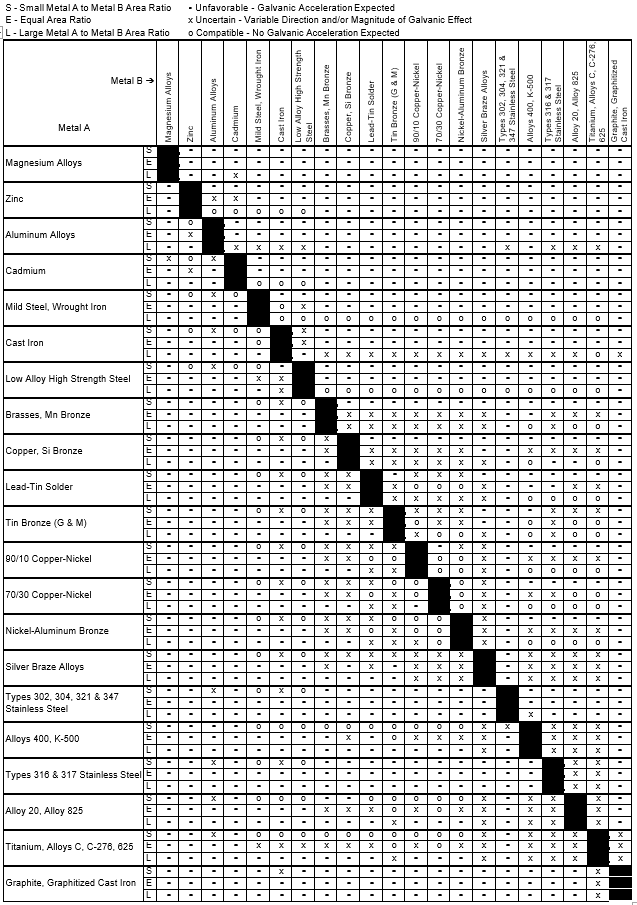
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master

Stainless Steel Galvanic Corrosion Chart
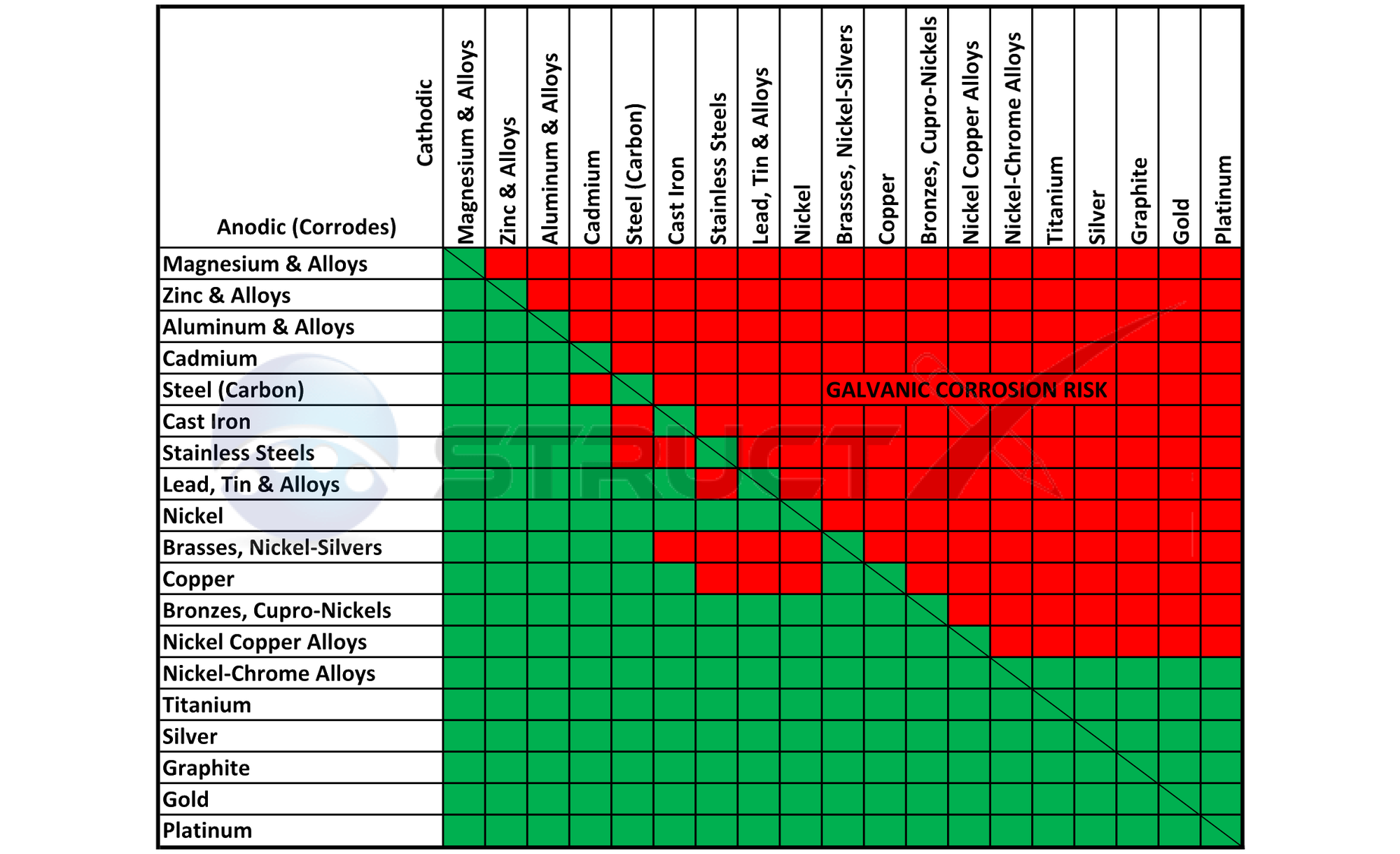
Aluminum Corrosion Resistance Chart
When Dissimilar Metals Are Used Together In The Presence Of An Electrolyte, Separate Them With A Dielectric Material Such As Insulation, Paint Or Similar Surface Coating.
Web Galvanic Corrosion Is An Electrochemical Process In Which One Metal Corrodes Preferentially To Another When Both Metals Are In Electrical Contact, In The Presence Of An Electrolyte.
The Table, However, Must Be Interpreted Not Only
Web Nec Sections 342.10 For Intermediate Metal Conduit (Imc), 344.10 For Rigid Metal Conduit (Rmc), And 358.10 For Electrical Metallic Tubing (Emt) Allow Galvanized Steel In Concrete, In Direct Contact With The Earth And Severely Corrosive Environments.
Related Post: