Chemistry Reaction Chart
Chemistry Reaction Chart - Web a combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The combustion of hydrogen gas. A chemical reaction is a process that occurs when two or more molecules interact to form a. Browse videos, articles, and exercises by topic. Web a chemical reaction is a process generally characterized by a chemical change in which the starting materials (reactants) are different from the products. Web a chemical reaction is a process in which one or more substances are converted to one or more different substances. Web these are the four prototypical organic chemistry reactions, though several others which can be categorized as one of these are generally referred to by other names. The starting substances are called the reactants, and the new substances that form are called the products. Combustion reactions must involve \(\ce{o_2}\) as one reactant. A free radical is a species with one (or more than one) unpaired electrons. If you look around, you’ll notice charts from different sources may order the elements slightly differently. A chemical reaction is a process or chemical change that transforms one set of substances ( the reactants) into another set of substances (the products ). A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. With. You can think of the reaction as swapping the cations or the anions, but not swapping both since you would end up with the same substances you started with. If you look around, you’ll notice charts from different sources may order the elements slightly differently. A reaction that occurs in two or more elementary steps is called a multistep or. Web the four main types of chemical reactions are synthesis, decomposition, single displacement, and double displacement reactions. Web the diagram shows heterolytic fission in which the most electronegative atom takes both electrons in the covalent bond and homolytic fission in which each atom takes one electron from the covalent bond. Chemical reactions tend to involve the motion of electrons, leading. Chemical reactions tend to involve the motion of electrons, leading to the formation and breaking of chemical bonds. Web to perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. Web one method of. To facilitate the finding of reactions from any of the keys that follow, a roadmap grid has been included. Web the rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient from the balanced equation. Web these are the four prototypical organic chemistry reactions, though several others. The remaining values will automatically be calculated. Look at these reactions and ask yourself this question for each: The reactivity series of metals is a list of metals arranged in their order of reactivity from highest to lowest. A chemical reaction is a process that occurs when two or more molecules interact to form a. A reaction that occurs in. Web this unit is part of the chemistry library. The five major types of chemical reactions are synthesis, decomposition, single replacement, double replacement, and combustion. A chemical reaction is a process that occurs when two or more molecules interact to form a. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a. With it being half term here in the uk, what better use of sudden vast amounts of free time could there be than making an organic chemistry reaction map? Chemical reactions tend to involve the motion of electrons, leading to the formation and breaking of chemical bonds. This graphic looks at simple interconversions between common functional groups in organic chemistry.. A free radical is a species with one (or more than one) unpaired electrons. If you look around, you’ll notice charts from different sources may order the elements slightly differently. Also, check ⇒ chemistry concept questions and answers. Web a chemical reaction is a process generally characterized by a chemical change in which the starting materials (reactants) are different from. Web click chemistry has become a commonly used synthetic method due to the simplicity, efficiency, and high selectivity of this class of chemical reactions. Web the rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient from the balanced equation. Web these are the four prototypical organic. A free radical is a species with one (or more than one) unpaired electrons. Web chemical reactions are classified into types to help scientists analyze them, and also to help scientists predict what the products of the reaction will be. Basic concepts of chemical reactions. This graphic looks at simple interconversions between common functional groups in organic chemistry. Web to perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. Web this unit is part of the chemistry library. Web let's summarize the key reactions of alcohols with this big reaction map [pdf] covering 67 reactions of alcohols, alkyl halides, alkenes, alkynes & more. Web the rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient from the balanced equation. The reactivity series of metals is a list of metals arranged in their order of reactivity from highest to lowest. To facilitate the finding of reactions from any of the keys that follow, a roadmap grid has been included. Also, check ⇒ chemistry concept questions and answers. At the bottom are the least reactive metals. Web here is an activity series chart for metals around room temperature. This is because the conditions of a proposed reaction matter. Plotting the log of the relative rate versus log of relative concentration provides information about the reaction. Web click chemistry has become a commonly used synthetic method due to the simplicity, efficiency, and high selectivity of this class of chemical reactions.
Types Of Chemical Reactions With Examples

Organic chemistry addition reactions chart Organic chemistry study

Types of Chemical Reactions Chemical reactions, Chemistry basics
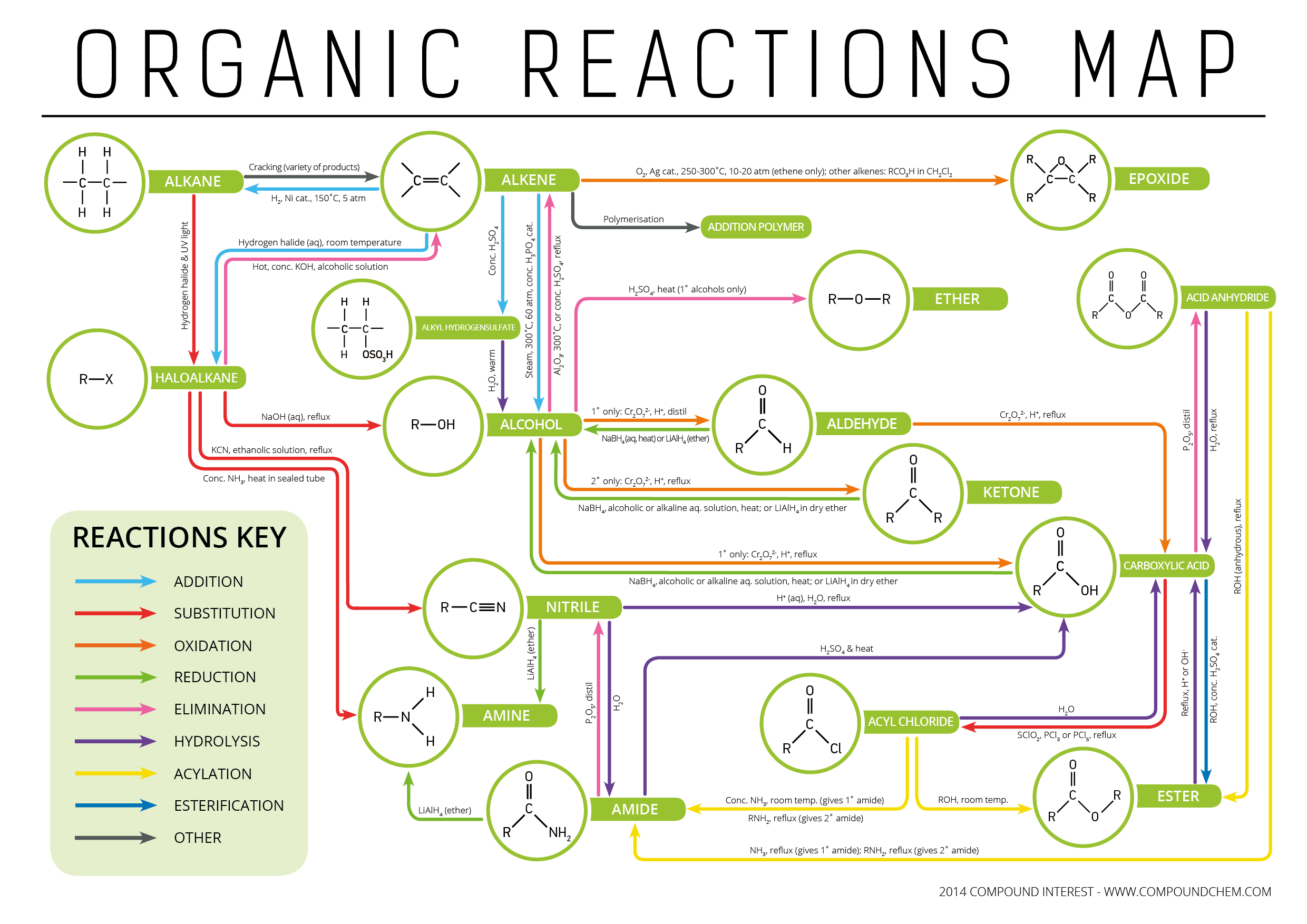
Types of Organic Reactions Functional Groups Interconversion

Reactions Alcohols and Carboxylic Acids Organic Chemistry Reactions

Chem Awareness Chemical Reactions
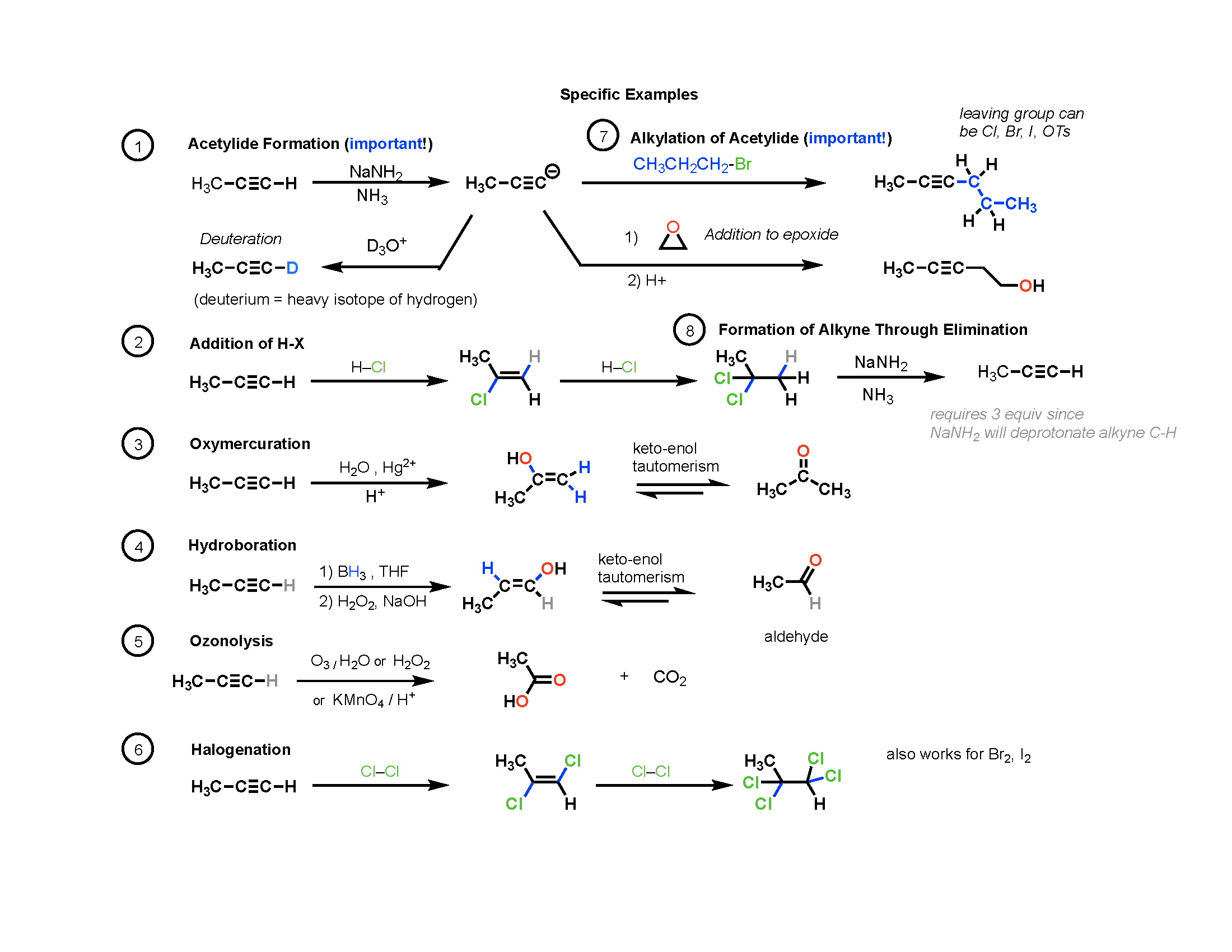
Reaction Maps Now Available Master Organic Chemistry
:max_bytes(150000):strip_icc()/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
Types of Chemical Reactions (With Examples)

Organic Chemistry Reactions Chart charts of organic chemistry reactions
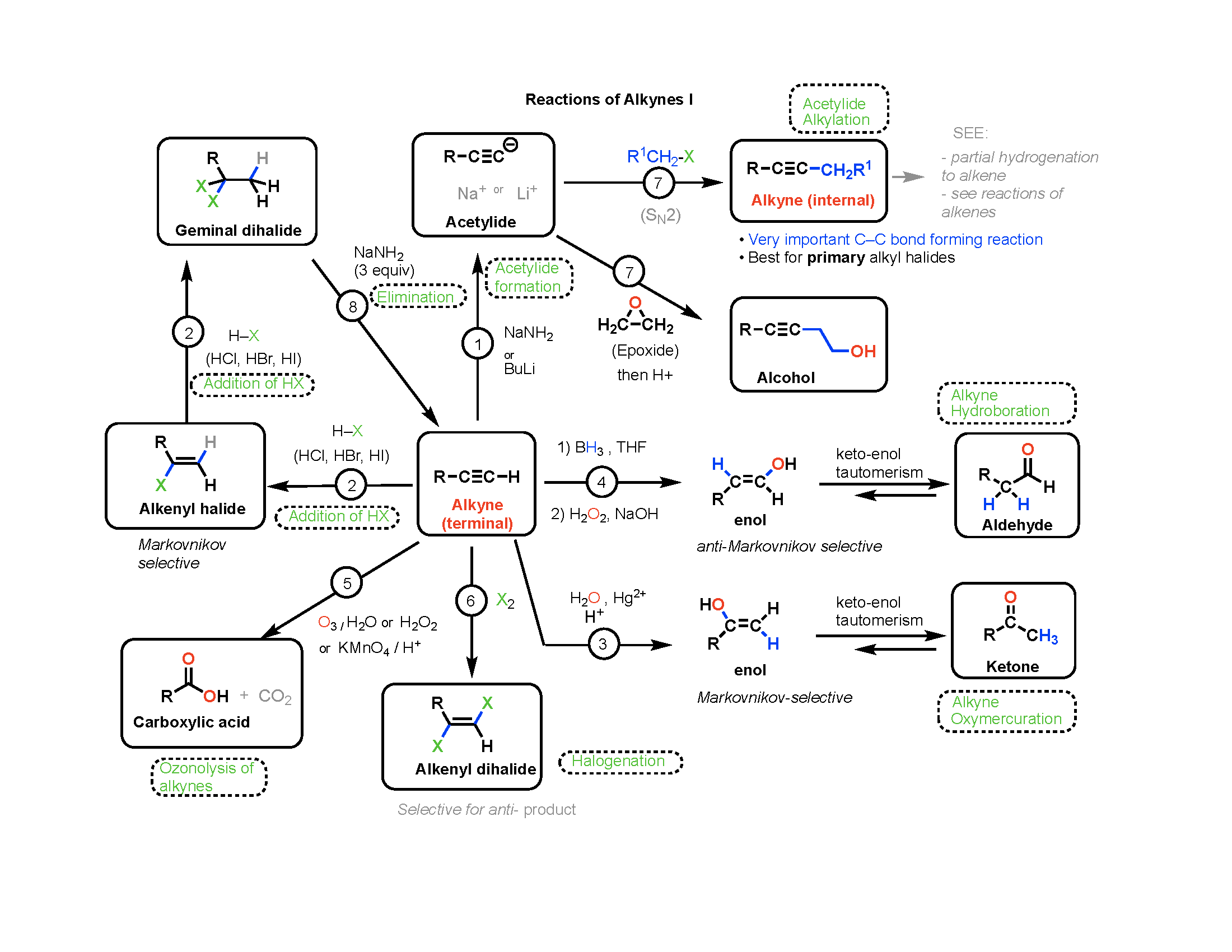
Reaction Maps Now Available Master Organic Chemistry
A Reaction Intermediate Is A Chemical Species That Is Formed In One Elementary Step And Consumed In A Subsequent Step.
Web A Chemical Reaction Is A Process In Which One Or More Substances Are Converted To One Or More Different Substances.
With It Being Half Term Here In The Uk, What Better Use Of Sudden Vast Amounts Of Free Time Could There Be Than Making An Organic Chemistry Reaction Map?
The Value Of N Is Not Related To The Reaction Stoichiometry And Must Be Determined By Experiment.
Related Post: