Stoichiometry Chart Conversions Chart
Stoichiometry Chart Conversions Chart - The mass of a substance and its number of moles are related through the conversion factor of m, the molar mass expressed in g/mol. To calculate a concentration, you need to divide the mass of the solute by the volume of the solvent. A à b (a and b can be any reactant or product) mass a à mol a mol a à mol b mol b à mass b (start) ga 1mola (start) mola (ratio) molb (start) molb mmb (g) mm a (g) (ratio) mola 1molb mol a ___ mol a mol b ___ mol b mass b ___ g b The quantitative relationship between the amounts of reactants consumed and those of products formed in chemical reactions as expressed by the balanced chemical equation for the reaction. The picture below is a representation of what i am saying. Web there are four steps in solving a stoichiometry problem: Web 1 mol fe 2 o 3: If you started with any unit other than moles, convert the amount to moles of “a” using dimensional analysis. A balanced chemical reaction can be used to determine molar and mass relationships between substances. The results generated by the tool should be considered for educational purposes only. 2.54 cm = 1 inch (exact) mass: The mass of a substance and its number of moles are related through the conversion factor of m, the molar mass expressed in g/mol. 454 g = 1 lb; The results generated by the tool should be considered for educational purposes only. As you work your way through chemistry problems use the map. Web stoichiometry conversion charts single conversions equation: Web flow chart for solving stoichiometry problems: Mass (g,kg, volume (l, or with. It can be used as a map for guiding you through these conversions. If you started with any unit other than moles, convert the amount to moles of “a” using dimensional analysis. Using this ratio, we could calculate how many moles of al are needed to fully react with a certain amount of fe 2 o 3 , or vice versa. Web versions of conversion tables below: Web the conversion factors are used to calculate the unknown quantity in the mole from the known quantity in the mole of any other reactant. You are notified to consult an expert in case you consider calculations as a reference anywhere. 1 gal = 3.7854 l. To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. Use the mole ratio to calculate the moles of wanted substance (b). Web common chemistry conversions english to metric conversions (mass, length,. A à b (a and b can be any reactant or product) mass a à mol a mol a à mol b mol b à mass b (start) ga 1mola (start) mola (ratio) molb (start) molb mmb (g) mm a (g) (ratio) mola 1molb mol a ___ mol a mol b ___ mol b mass b ___ g b In. 2.54 cm = 1 inch (exact) mass: Web 1 pint = 2 cups = 16 fluid ounces. 454 g = 1 lb; N2(g) +3h2(g) → 2nh3(g) mole of n2 and 3 moles of h2 can make 2 moles of nh3. Web mass and grade conversion factors 1 troy oz 31.1035g 1g 0.03215 troy oz 1 short ton 2000lb 907.2kg 0.9072t. Web this means that the mole conversions for stoichiometry are the most important conversions to learn. Web the conversion factors are used to calculate the unknown quantity in the mole from the known quantity in the mole of any other reactant or product in the same chemical equation, as explained in the following examples. Web stoichiometry conversion charts single conversions. Web stoichiometry tutorial for converting mass and moles using stoichiometric conversions, balanced reactions, and molecular weights. Write the balanced chemical equation. Web 1 pint = 2 cups = 16 fluid ounces. Web 1 mol fe 2 o 3: Web the conversion factors are used to calculate the unknown quantity in the mole from the known quantity in the mole of. Upon establishing that a stoichiometric molar relationship is required to solve a problem, a corresponding stoichiometric equality must be developed. Web stoichiometry conversion chart mass mass moles moles use molar ratio from balanced volume chemical equation. Web you will see a selection of chart images that show different aspects of chemistry stoichiometry conversion chart, such as stoichiometry flowchart chemical conversions,. 454 g = 1 lb; Web you will see a selection of chart images that show different aspects of chemistry stoichiometry conversion chart, such as stoichiometry flowchart chemical conversions, how do you solve a stoichiometry problem example, smart exchange usa stoichiometry conversion chart, and more. Web in chemistry the most common way to describe concentration is with molarity (mol/l). Then,. The picture below is a representation of what i am saying. In ap/college you won't see these because we need students to be able to understand dimensional analysis (conversions) and stoichiometry. 1 lb = 16 oz = 453.6 g. Stoichiometry conversion chart created date:. Web stoichiometry tutorial for converting mass and moles using stoichiometric conversions, balanced reactions, and molecular weights. Convert the units of the given substance (a) to moles. In general, mole ratios can be used to convert between amounts of any two substances involved in a chemical reaction. N2(g) +3h2(g) → 2nh3(g) mole of n2 and 3 moles of h2 can make 2 moles of nh3. Web there are four steps in solving a stoichiometry problem: If your honors / pap chemistry teacher is preparing students for any ap science (not just chemistry) they. Web flow chart for solving stoichiometry problems: In other words, stoichiometry is used to figure out if you’ve got enough crackers to make 30 sandwiches, or how much cheese you’ll need to make 15 sandwiches. Web common chemistry conversions english to metric conversions (mass, length, volume, and area. Web stoichiometry conversion chart mass mass moles moles use molar ratio from balanced volume chemical equation. You will also discover how to use. Web apply multiple conversion factors to convert between a mass and a particle count of two substances that participate in a chemical reaction.
Molecular Weight and Stoichiometry Explained in Simple Steps

Stoichiometry Lessons TES chemistry & physics Pinterest

The Stoichiometric Chart YouTube

Extended Reaction Stoichiometry Road Map — Examples Expii
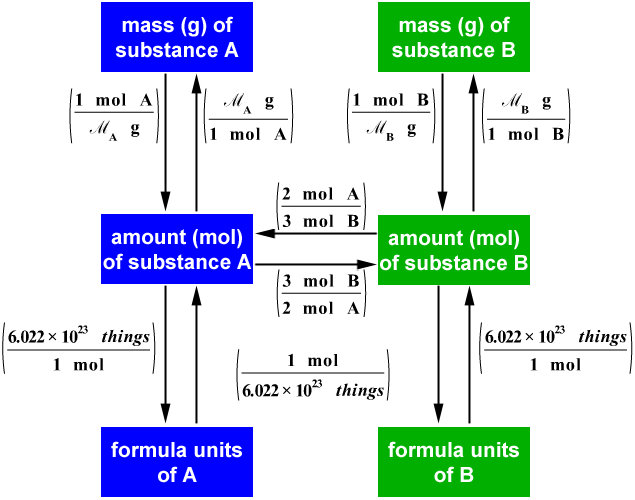
C&J&S&B's Class Chemisty ) What is Stoichiometry?

Stoichiometry Introduction Using Stoich Tables YouTube
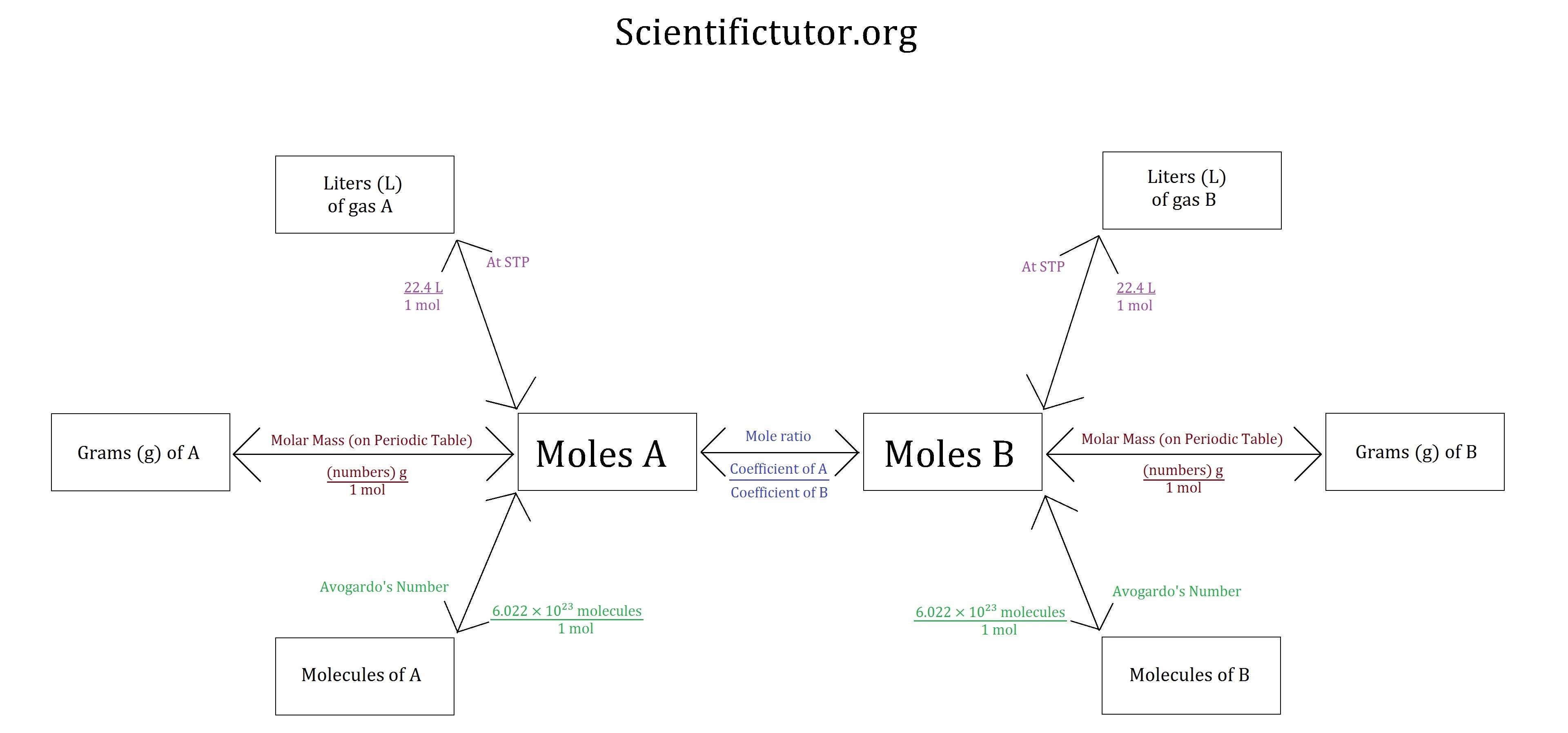
Chem Gas Stoichiometry Scientific Tutor
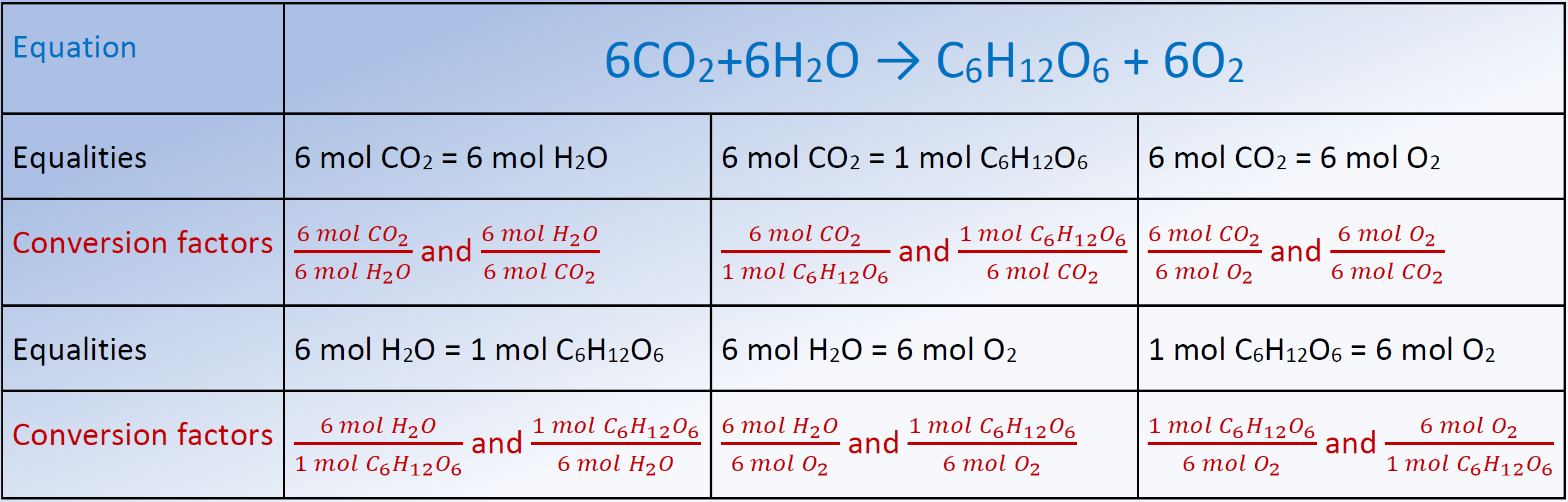
Chemistry Conversion Chart Moles
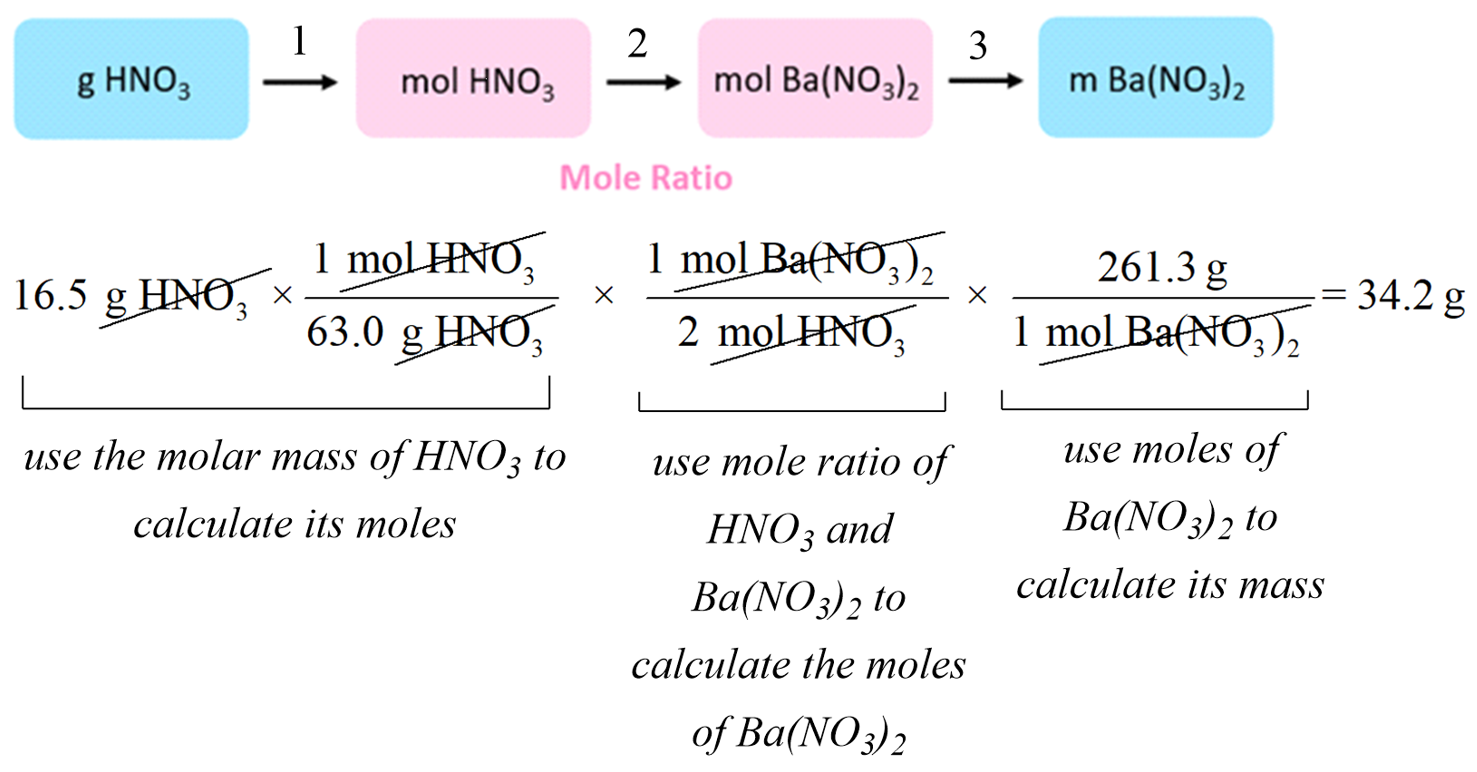
Stoichiometry of Chemical Reactions Chemistry Steps
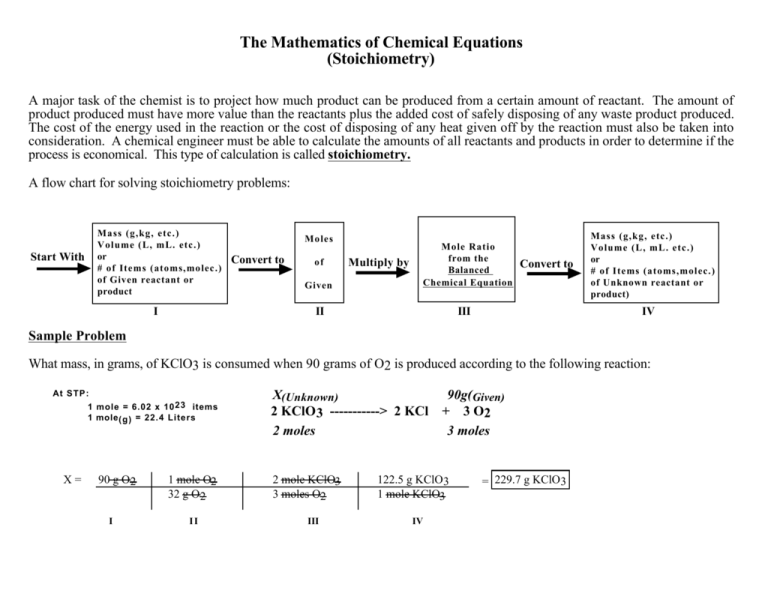
Stoichiometry Conversions Chart chegos.pl
Web Stoichiometry Is A Set Of Calculations You Perform To Figure Out How Much Stuff You Can Make In A Reaction, Or How Much Stuff You Will Need To Make The Reaction Occur.
Web 1 Mol Fe 2 O 3:
Using This Ratio, We Could Calculate How Many Moles Of Al Are Needed To Fully React With A Certain Amount Of Fe 2 O 3 , Or Vice Versa.
Web Chemistry English To Metric Conversion Chart Bmpo.
Related Post: