Red Cabbage Indicator Color Chart
Red Cabbage Indicator Color Chart - Web in this activity you will use red cabbage to make what is called an indicator solution. Is a substance that changes colour when it is added to acidic or alkaline solutions. With colors ranging from red to pink to violet to blue to green, they can brighten up any discussion of acids and bases. Web cabbage juice changes to red in the presence of an acid (ph less than 7), is blue at neutral ph (ph around 7), and is purple in the presence of a base (ph greater than 7). Red cabbage juice indicators are easy to make, exhibit a wide range of colors, and can be used to make your own ph paper strips. Ph more than 7 = base. The ph scale goes from 0 to 14. Pure water is neutral, and so is petrol. The pigment molecule flavin or anthocyanin found in cabbage changes colour based on the solution’s acidity. Red cabbage (3 cups) knife or blender. By anne marie helmenstine, ph.d. Make an acid/base indicator solution from red cabbage to test the ph of different household solutions. In this case adding something acidic (like lemon juice) will change it to one color while adding something basic (like bleach) will change it to another. These diferent structures give a range of. The pigments in red cabbage are. Web in this activity you will use red cabbage to make what is called an indicator solution. Each carbon has 4 bonds. Various colouring materials in plants can act as indicators. Web cabbage juice changes to red in the presence of an acid (ph less than 7), is blue at neutral ph (ph around 7), and is purple in the. Just sprinkle in a little lime juice and your previously purple cabbage will turn pink! Web in this activity you will use red cabbage to make what is called an indicator solution. The structures of the anthocyanin pigments which give the red cabbage its colour are subtly changed at varying ph. With colors ranging from red to pink to violet. Make an acid/base indicator solution from red cabbage to test the ph of different household solutions. A substance with a ph of 0 is a strong acid, ph 14 is a strong alkali, and ph 7 is neutral. Web it provides simple instructions for making and testing red cabbage indicator paper, and includes a colour comparison chart. By anne marie. If you use a blender, add the minimum amount of water you need. Ph more than 7 = base. Web chart of common ph indicators. Web cabbage juice changes to red in the presence of an acid (ph less than 7), is blue at neutral ph (ph around 7), and is purple in the presence of a base (ph greater. Indicator solutions can change colors depending on what you add to them. In this case adding something acidic (like lemon juice) will change it to one color while adding something basic (like bleach) will change it to another. Web your red cabbage indicator should be dark blue in color. Web cabbage juice changes to red in the presence of an. You can make an indicator using red cabbage. These diferent structures give a range of. Red cabbage, commonly found in homes, can make a ph indicator solution. A substance with a ph of 0 is a strong acid, ph 14 is a strong alkali, and ph 7 is neutral. O liquid cleaning products (don’t use bleach) o solutions made by. Web red cabbage juice contains a natural ph indicator that changes colors according to the acidity of the solution. Web chart of common ph indicators. The salt in the salt water has dissolved and the solution looks clear, but the salt is still there and will taste salty if you taste it. Web an indicator will change colour in the. Just sprinkle in a little lime juice and your previously purple cabbage will turn pink! Red cabbage juice indicators are easy to make, exhibit a wide range of colors, and can be used to make your own ph paper strips. A ph indicator is a substance which has one colour when added to an acidic solution and a different colour. The salt in the salt water has dissolved and the solution looks clear, but the salt is still there and will taste salty if you taste it. Web unlike most other natural ph indicators, cabbage juice displays a wide range of colors. Red or purple cabbage juice is. If you use a blender, add the minimum amount of water you. The salt in the salt water has dissolved and the solution looks clear, but the salt is still there and will taste salty if you taste it. The colour of the leaves is determined by the ph of the soil. The pigments in red cabbage are excellent examples of natural ph indicators. 15 minutes plus drying time. O fruit juice, for example: Web your red cabbage indicator should be dark blue in color. Want to give your red cabbage salad a nice vibrant pinkish color? Learn more about downloading digital content. Web in this activity you will use red cabbage to make what is called an indicator solution. Just sprinkle in a little lime juice and your previously purple cabbage will turn pink! Red cabbage is a natural indicator helps in determining the ph of soil. A substance with a ph of 0 is a strong acid, ph 14 is a strong alkali, and ph 7 is neutral. Web yellow green (at ph >8) hydrogens on carbon atoms implied; Chop or blend a red or purple cabbage. With colors ranging from red to pink to violet to blue to green, they can brighten up any discussion of acids and bases. If you use a blender, add the minimum amount of water you need.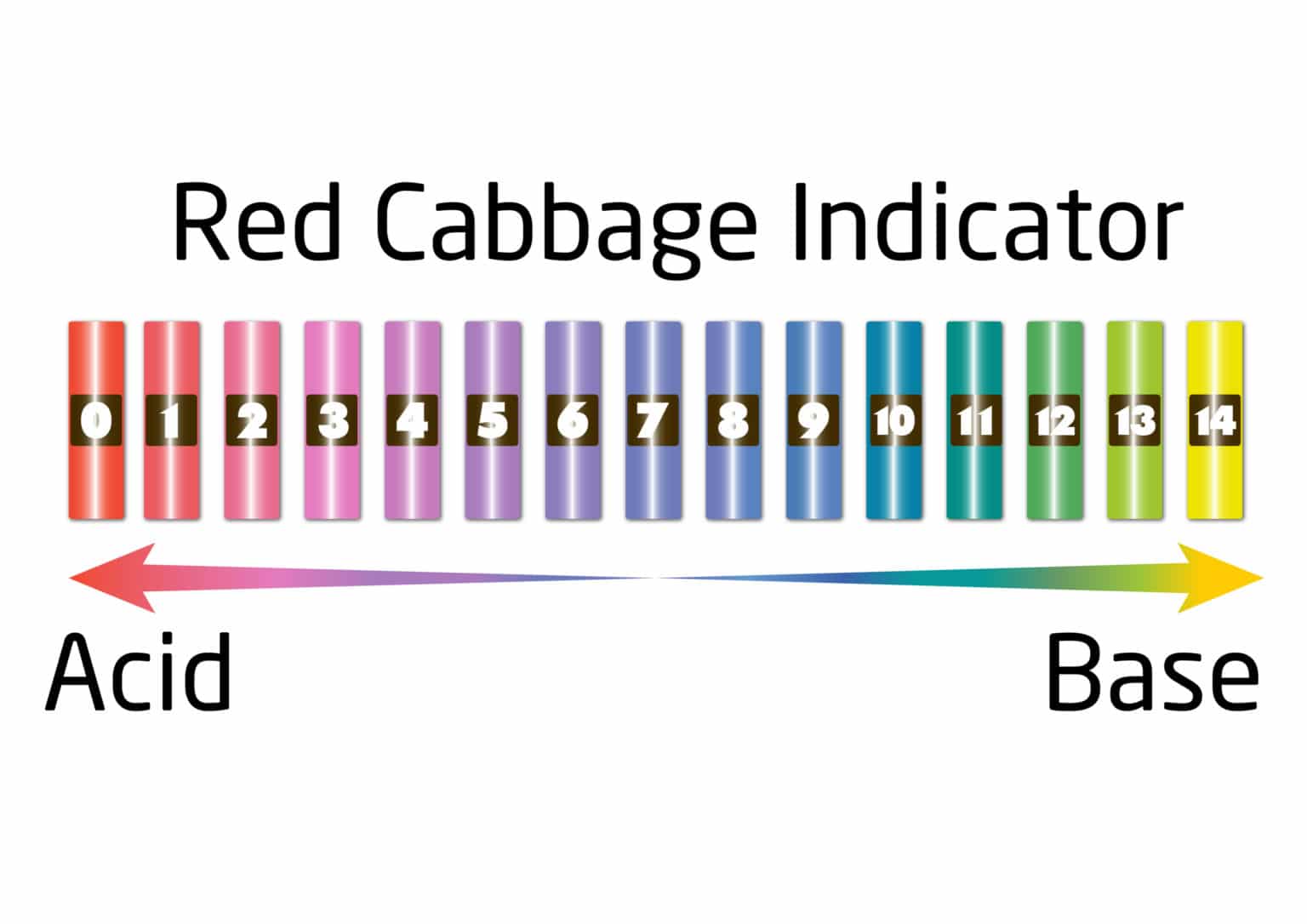
How to make a red cabbage pH indicator Chemistry for Kids

Red Cabbage Indicator Chemistry Demonstrations

pH Indicator from Red Cabbage Red cabbage, Cabbage and Ph
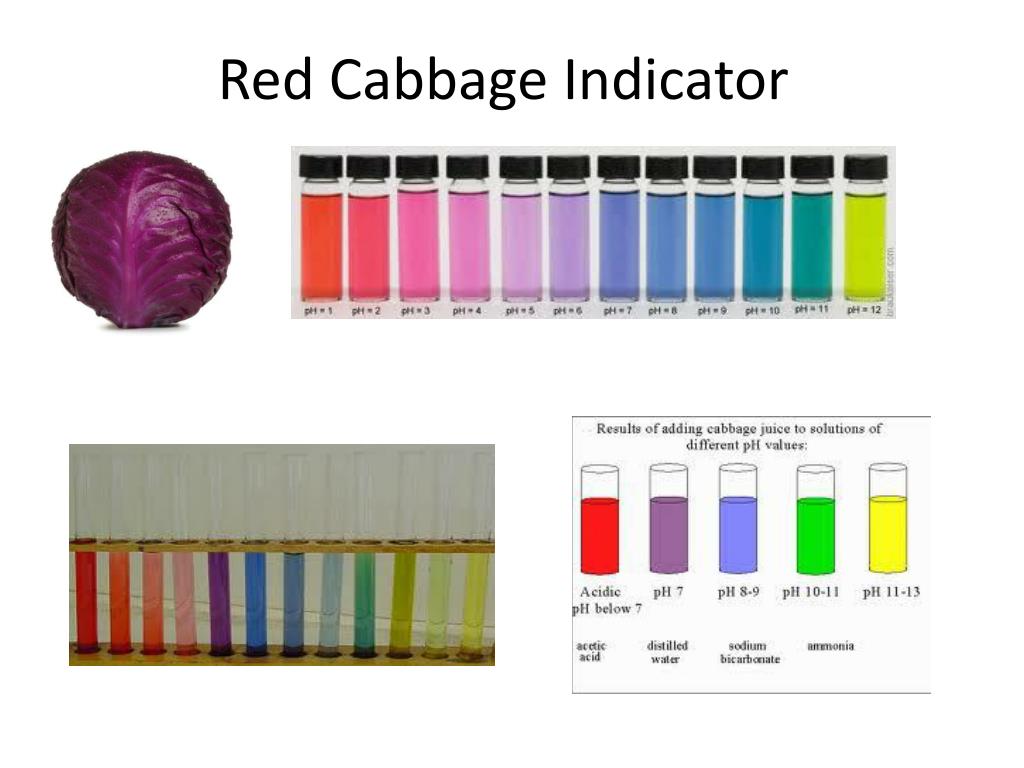
PPT Red Cabbage Indicator PowerPoint Presentation, free download ID

Red cabbage dye (and pH indicator) ingridscience.ca
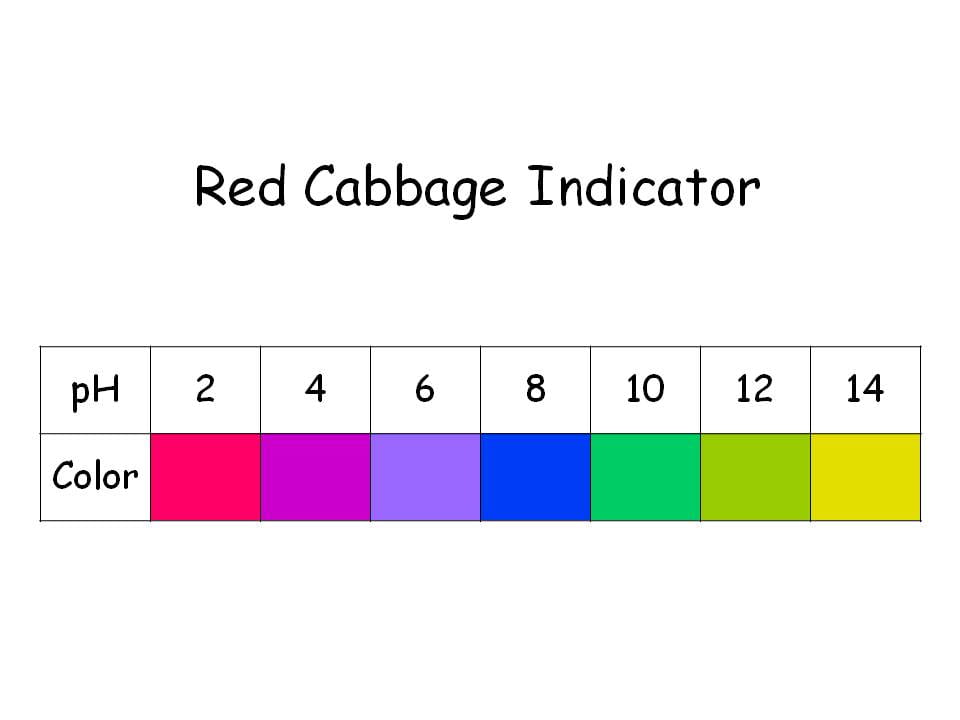
Red Cabbage Indicator Chemistry Demonstrations

Acid and Base Lab with Report The Art of Science
![]()
Natural pH Indicators Make a pH indicator using beetroot or cabbage!

Red cabbage dye (and pH indicator) ingridscience.ca
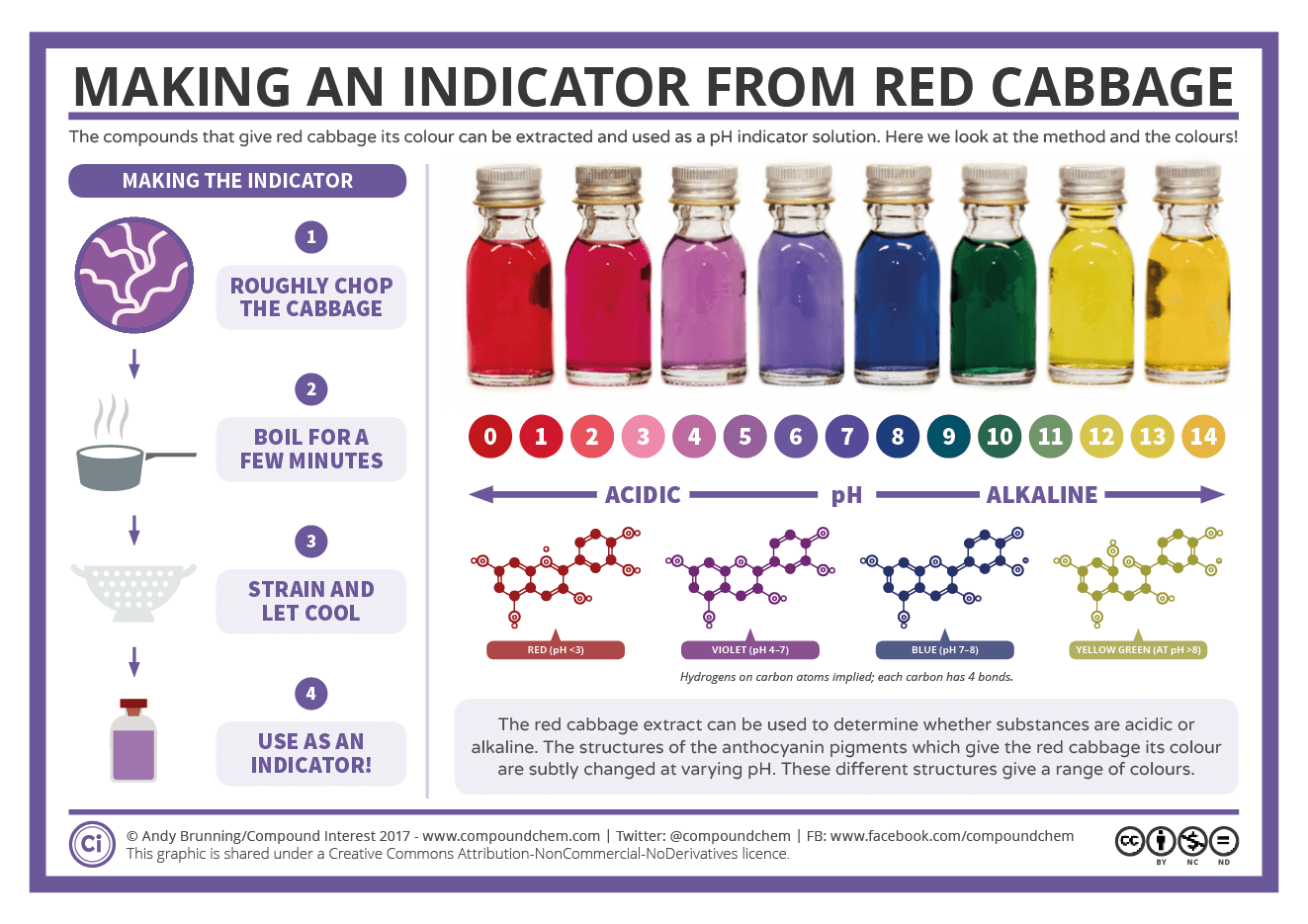
Making a Red Cabbage pH Indicator The Method and the Chemistry
Web Red Cabbage Color Indicator Chart !!
Web It Provides Simple Instructions For Making And Testing Red Cabbage Indicator Paper, And Includes A Colour Comparison Chart.
You Can Make An Indicator Using Red Cabbage.
O Liquid Cleaning Products (Don’t Use Bleach) O Solutions Made By Dissolving A Solid Such As Baking Soda, Detergent, Or Baking Powder In Water.
Related Post: