Pka Chart Of Amino Acids
Pka Chart Of Amino Acids - A homogeneous substance, something that your diet should contain in. Web if you were to synthesize enantiomers (like amino acids) in a laboratory, you'd find that it's possible to get a 50/50 production of 'l' and 'd' enantiomers depending on your reaction mechanism. Web pka is an acid dissociation constant used to describe the acidity of a particular molecule. Summary of pkas of amino acids Web each amino acid has its own pi value based on the properties of the amino acid. Amino acids are a crucial, yet basic unit of protein, and they contain an amino group and a carboxylic group. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. To learn the structures, names, and shorthand, the best method here is memorization. We tend to think of protein as a mass noun: For the three amino acids with a basic side chain, p i is the average of the two highest p ka values. The structure and properties of amino acids. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. That is a daunting task for 20 amino acids. Web all amino acids have the same basic structure, which is shown in figure 2.1. The isoelectric points range from 5.5 to 6.2. 3 pkx is the negative of the logarithm of the dissociation constant for any other group in the molecule. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal genetic code. For the three amino acids with a basic side chain, p i is the average of the two highest. Web with only very minor exceptions, every amino acid found in cells and in proteins is in the l configuration. In organic chemistry, pka is a measure of the acidity or basicity of a compound. Web all amino acids have the same basic structure, which is shown in figure 2.1. Titration curves show the neutralization of these acids by added. Web with only very minor exceptions, every amino acid found in cells and in proteins is in the l configuration. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal genetic code. The isoelectric points range from 5.5 to 6.2. The structure and properties of amino acids. Web the pka. Web the pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. Web if you were to synthesize enantiomers (like amino acids) in a laboratory, you'd find that it's possible to get. We tend to think of protein as a mass noun: Web amino acids reference chart. Amino acids are a crucial, yet basic unit of protein, and they contain an amino group and a carboxylic group. You will learn how to calculate the isoelectric point, and the effects of ph on the amino acid's overall charge. 3 pkx is the negative. For the three amino acids with a basic side chain, p i is the average of the two highest p ka values. At ph values above or below the isoelectric point, the molecule will have a net charge which depends on its pi value as well as the ph of the solution in which the amino acid is found. In. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. Web table of pka and pi values. Web for amino acids with acidic sidechains, the pi can be calculated by averaging the pk a values of the two most acidic groups. Web table \(\pageindex{2}\) shows the standard pk a values for the amino acids and can. If you want to know more about this, you can view the lecture series on organic chemistry as it is elaborated further there. Web all amino acids have the same basic structure, which is shown in figure 2.1. A homogeneous substance, something that your diet should contain in. Properties of common amino acids. That is a daunting task for 20. Summary of pkas of amino acids Web table \(\pageindex{2}\) shows the standard pk a values for the amino acids and can be used to predict the ionization/charge status of amino acids and their resulting peptides/proteins. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. It represents the negative logarithm of the acid dissociation constant (ka),. You will learn how to calculate the isoelectric point, and the effects of ph on the amino acid's overall charge. Web each amino acid has its own pi value based on the properties of the amino acid. Web pka is an acid dissociation constant used to describe the acidity of a particular molecule. Web most biochemistry courses will require you to know the following: If you want to know more about this, you can view the lecture series on organic chemistry as it is elaborated further there. There are 22 amino acids that are found in proteins and of these, only 20 are specified by the universal genetic code. Web table of pka and pi values. Web for amino acids with acidic sidechains, the pi can be calculated by averaging the pk a values of the two most acidic groups. Web the pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. 3 pkx is the negative of the logarithm of the dissociation constant for any other group in the molecule. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. The structure and properties of amino acids. Web amino acids reference chart. We tend to think of protein as a mass noun: The isoelectric points range from 5.5 to 6.2. Summary of pkas of amino acids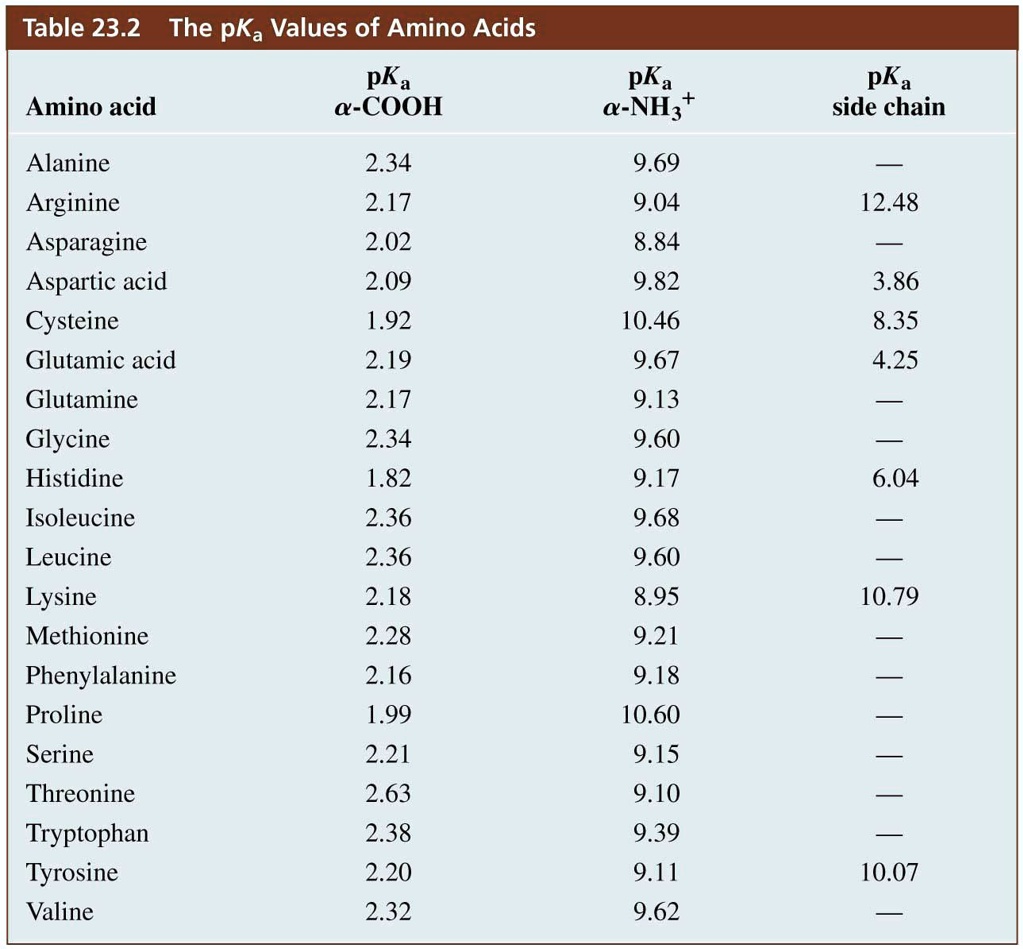
SOLVED Table 23.2 The pKa Values of Amino Acids Amino Acid pKa M
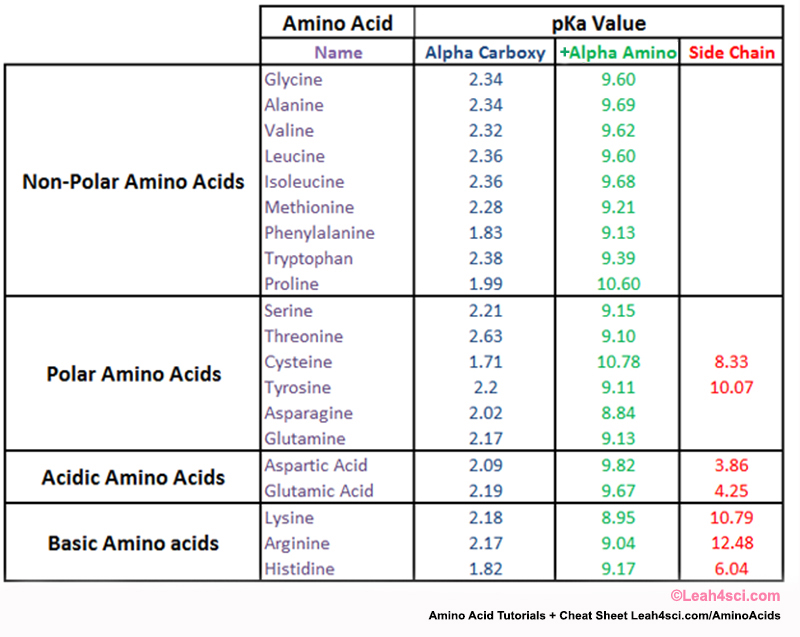
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial

The pKa in Organic Chemistry Chemistry Steps
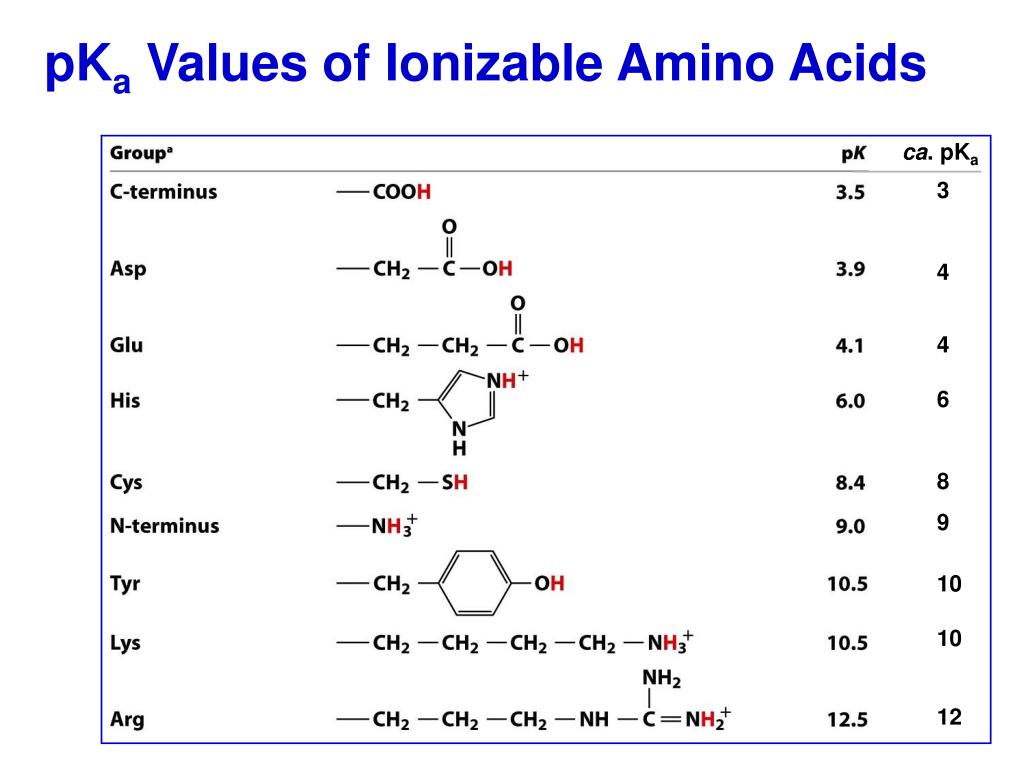
Amino Acids Pka Chart
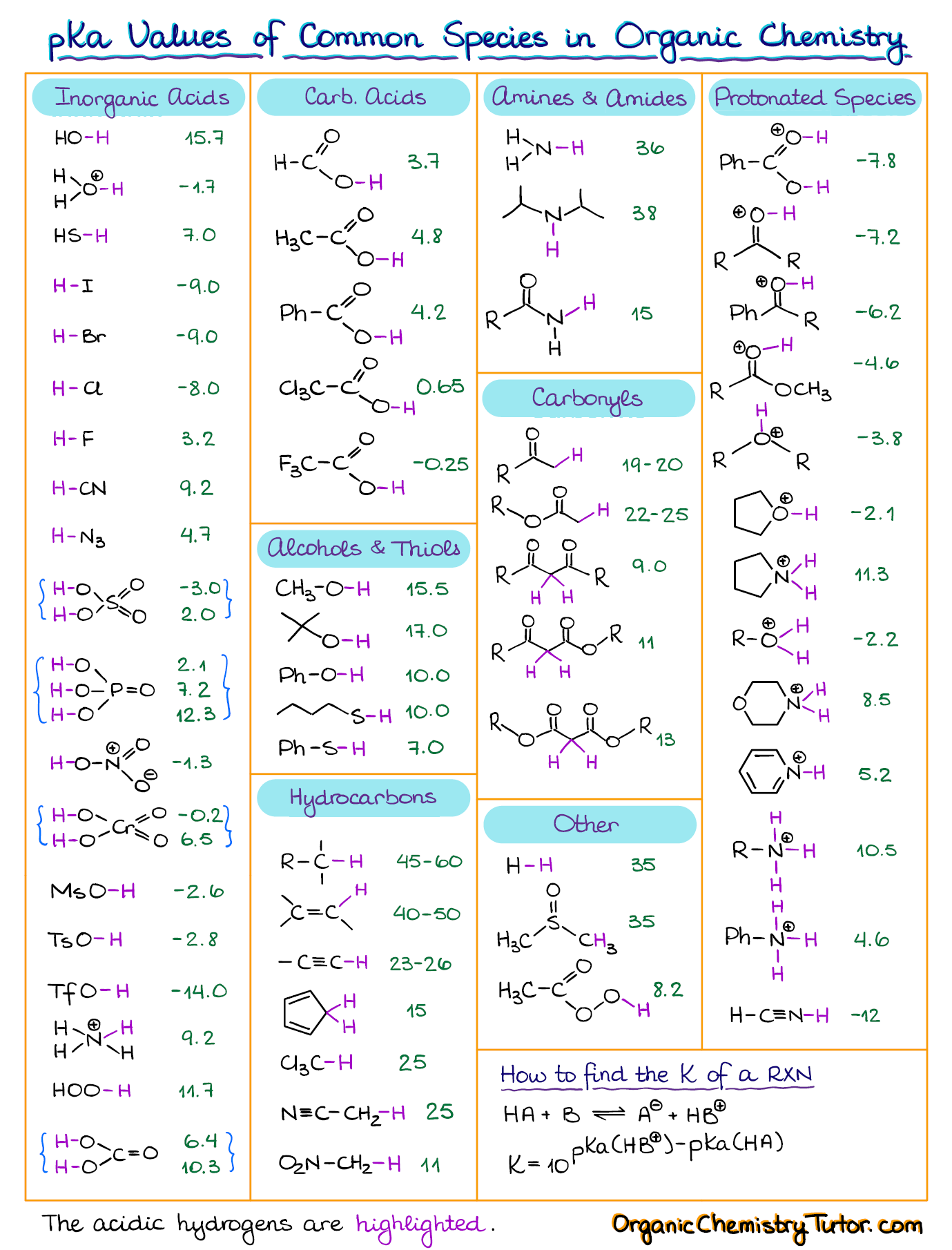
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic
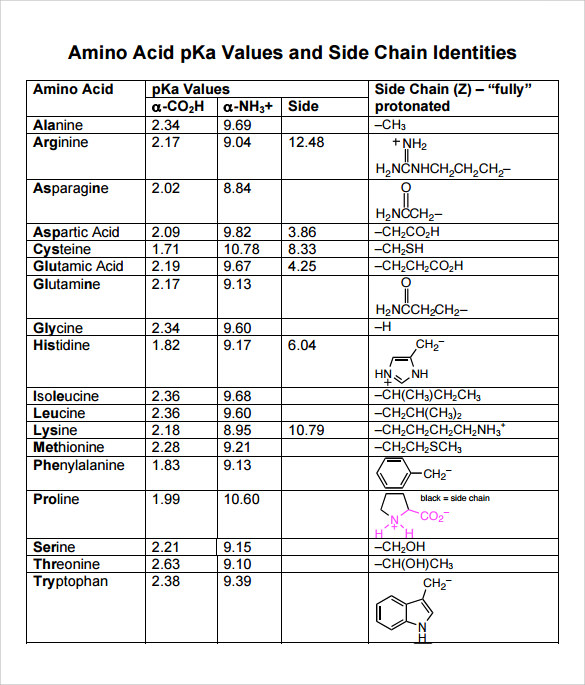
Pka Chart Amino Acids
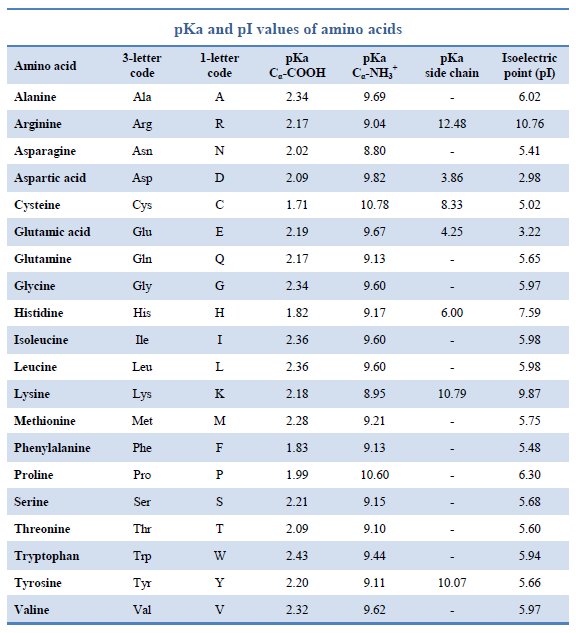
Amino acid properties
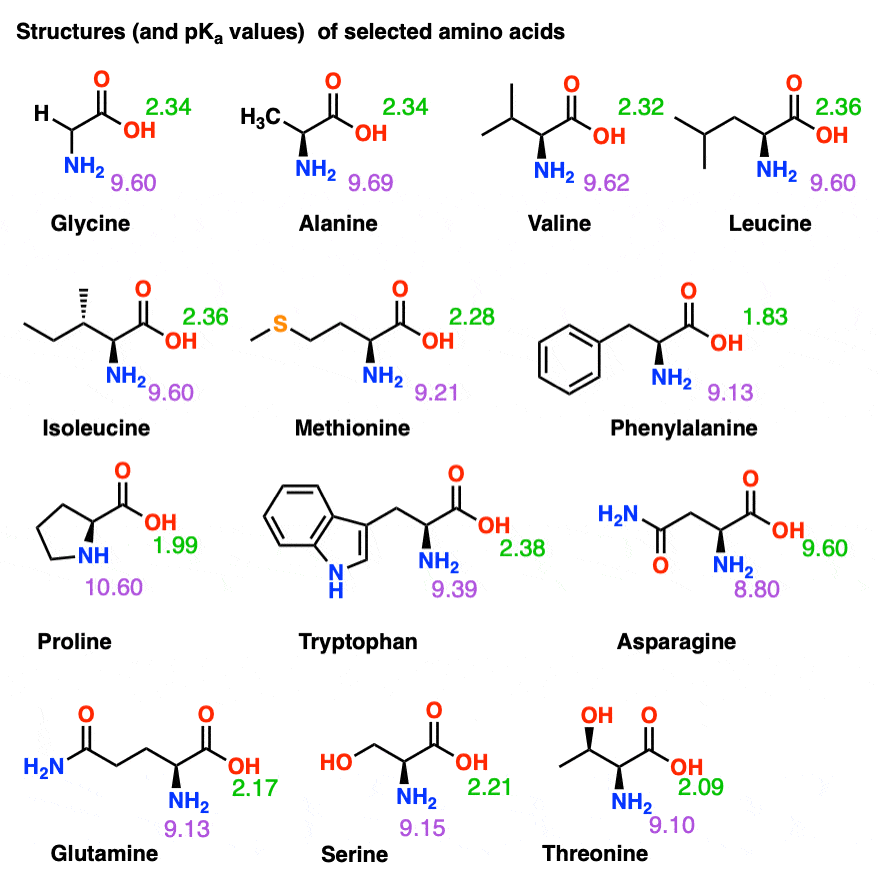
Isoelectric Points of Amino Acids (and How To Calculate Them) Master

Pka Values Of Amino Acids
![[Infographic] Comprehensive pKa Chart r/chemistry](https://external-preview.redd.it/K3Snfd3HbLKkQbUsJ8g5GMVBN8te4Altg0_bder8QLE.jpg?auto=webp&s=d6f9b865541ac9cf9c578f5146e380a014396caa)
[Infographic] Comprehensive pKa Chart r/chemistry
Its Value Is Directly Related To The Structure Of The Given Compound.
We Will Also Discuss Zwitterions, Or The Forms Of Amino Acids That Dominate At The Isoelectric Point.
Web With Only Very Minor Exceptions, Every Amino Acid Found In Cells And In Proteins Is In The L Configuration.
Titration Curves Show The Neutralization Of These Acids By Added Base, And The Change In Ph During The Titration.
Related Post: