Pka Chart Amino Acids
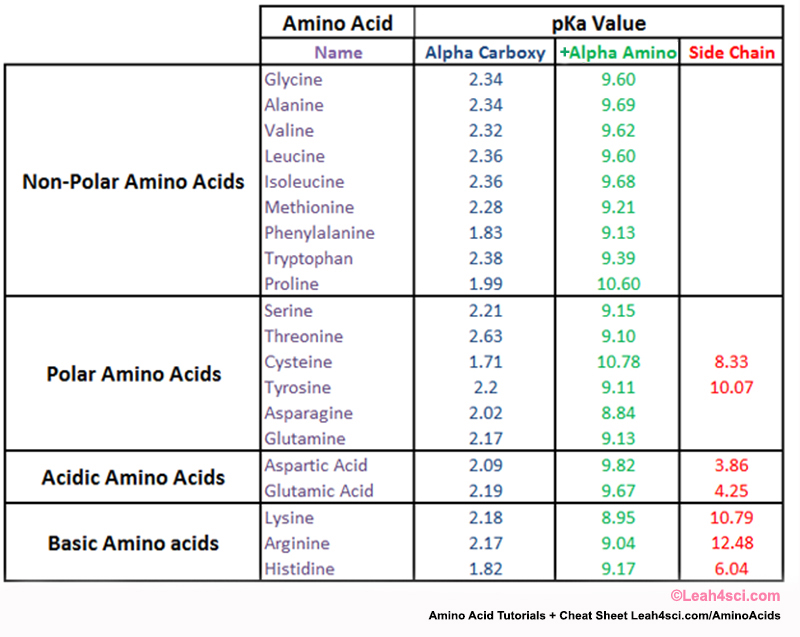
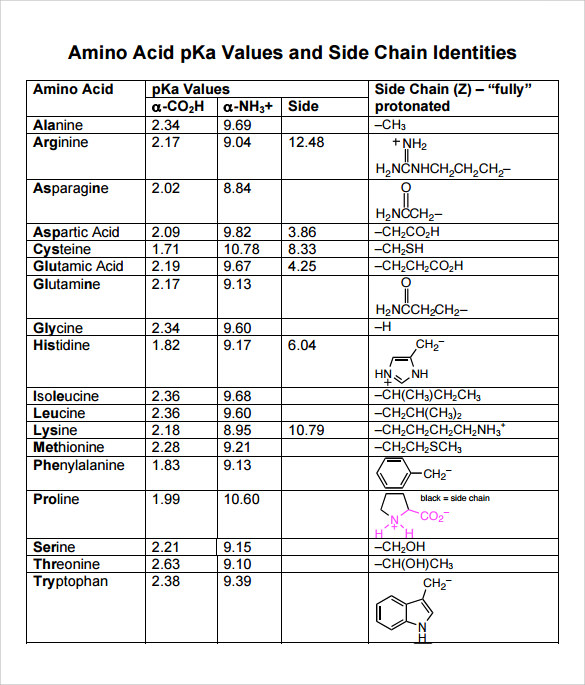
Pka Chart Amino Acids - Amino acids are a crucial, yet basic unit of protein, and they contain an amino group and a carboxylic group. Web the pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. We will also discuss zwitterions, or the forms of amino acids that dominate at the isoelectric point. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. The isoelectric points range from 5.5 to 6.2. Web you should be able to classify all the amino acids by polarity, charge, aliphatic vs aromatic, and probably learn the structures and functional groups of the special amino acids (for example: They contain an amino group, carboxylic acid group, alpha carbon, and side chain. For the 13 amino acids with a neutral side chain, p i is the average of p k a1 and p k a2. 3 pkx is the negative of the logarithm of the dissociation constant for any other group in the molecule. They play an extensive role in gene expression process, which includes an adjustment of protein functions that facilitate messenger rna (mrna). Two cysteines close in space may form disulfide bridges under oxidizing conditions, prolines tend to introduce kinks in polypeptides and are often found at. Web the pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. 3 pkx is the negative of the logarithm of the dissociation constant for any other. The side chains of acid and basic amino acids, and some polar amino acids can also be titrated: The r group side chains may be either nonpolar, polar and uncharged, or charged, depending on the functional group, the ph, and the pka of any ionizable group in the side chain. To learn the structures, names, and shorthand, the best method. They contain an amino group, carboxylic acid group, alpha carbon, and side chain. Most amino acids have a chiral carbon, which allows them to rotate polarized light. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. To learn the structures, names, and shorthand, the best method here is memorization. Conjugate. Conjugate acid of − nh 2, i.e., − nh + 3 has pk a ~10. Web amino acid pka values. Web amino acids reference chart. Properties of common amino acids. Web amino acid reference chart contains the twenty amino acids found in eukaryotes, grouped according to their side chains and charge. Web all amino acids have the same basic structure, which is shown in figure 2.1. Web pka is an acid dissociation constant used to describe the acidity of a particular molecule. Most amino acids have a chiral carbon, which allows them to rotate polarized light. Created by tracy kim kovach. Web the isoelectric point of an amino acid is the. Web the isoelectric point of an amino acid is the ph at which the amino acid has a neutral charge. Two cysteines close in space may form disulfide bridges under oxidizing conditions, prolines tend to introduce kinks in polypeptides and are often found at. Web the pka is a measure of the strength of an acid, i.e., the lower the. For the 13 amino acids with a neutral side chain, p i is the average of p k a1 and p k a2. Web amino acid reference chart contains the twenty amino acids found in eukaryotes, grouped according to their side chains and charge. Two cysteines close in space may form disulfide bridges under oxidizing conditions, prolines tend to introduce. The isoelectric points range from 5.5 to 6.2. The r group side chains may be either nonpolar, polar and uncharged, or charged, depending on the functional group, the ph, and the pka of any ionizable group in the side chain. Its value is directly related to the structure of the given compound. For the 13 amino acids with a neutral. The r group side chains may be either nonpolar, polar and uncharged, or charged, depending on the functional group, the ph, and the pka of any ionizable group in the side chain. Its value is directly related to the structure of the given compound. Most amino acids have a chiral carbon, which allows them to rotate polarized light. Web the. Web amino acid pka values. Web the pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. The r group side chains may be either nonpolar, polar and uncharged, or charged, depending on the functional group, the ph, and the pka of any ionizable group in the side chain. Thus, amino. Two cysteines close in space may form disulfide bridges under oxidizing conditions, prolines tend to introduce kinks in polypeptides and are often found at. Thus, amino acids with (chemically) similar side groups can be expected to function in. The r group, which differs for each amino. For the 13 amino acids with a neutral side chain, p i is the average of p k a1 and p k a2. They play an extensive role in gene expression process, which includes an adjustment of protein functions that facilitate messenger rna (mrna). Web the isoelectric point of an amino acid is the ph at which the amino acid has a neutral charge. You will learn how to calculate the isoelectric point, and the effects of ph on the amino acid's overall charge. The isoelectric points range from 5.5 to 6.2. Web you should be able to classify all the amino acids by polarity, charge, aliphatic vs aromatic, and probably learn the structures and functional groups of the special amino acids (for example: Properties of common amino acids. Created by tracy kim kovach. Most amino acids have a chiral carbon, which allows them to rotate polarized light. Web pka is an acid dissociation constant used to describe the acidity of a particular molecule. We will also discuss zwitterions, or the forms of amino acids that dominate at the isoelectric point. Web the pka is a measure of the strength of an acid, i.e., the lower the pk a stronger the acid. Web all amino acids have the same basic structure, which is shown in figure 2.1.
Pka Values Of Amino Acids
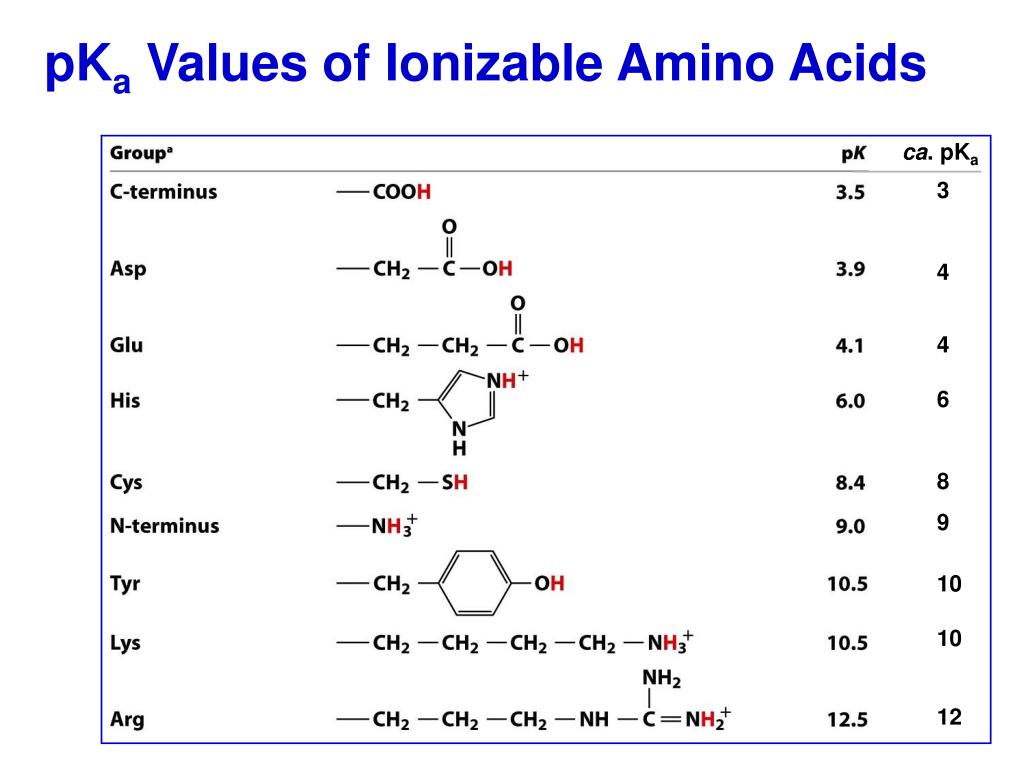
Amino Acids Pka Chart
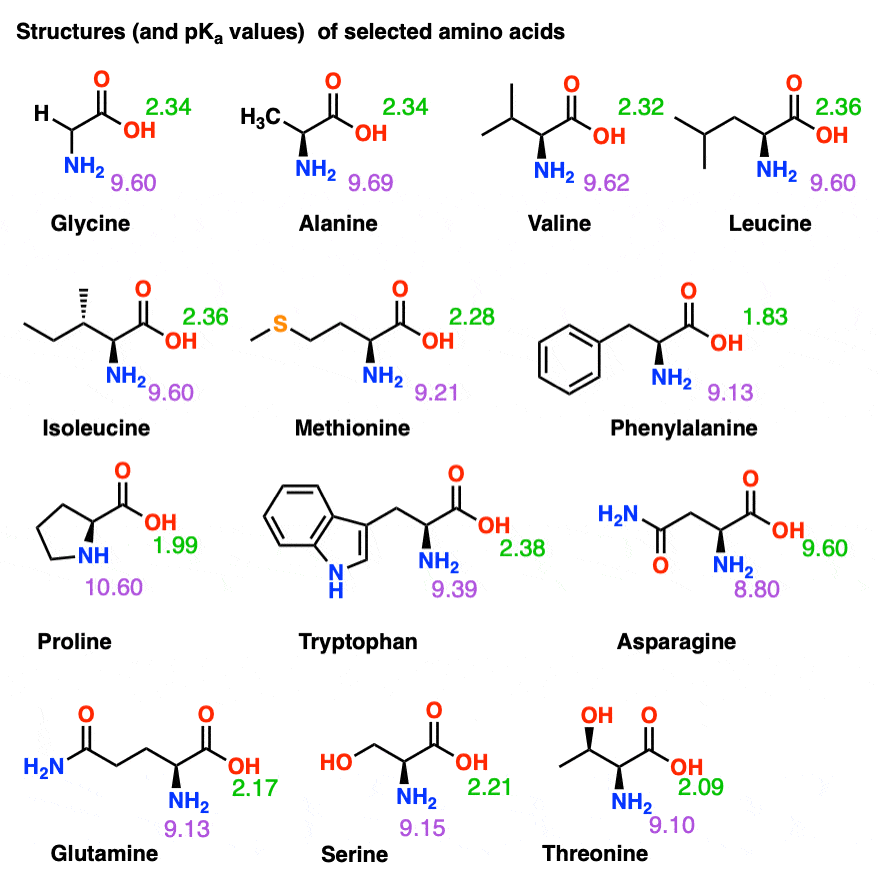
Isoelectric Points of Amino Acids (and How To Calculate Them) Master
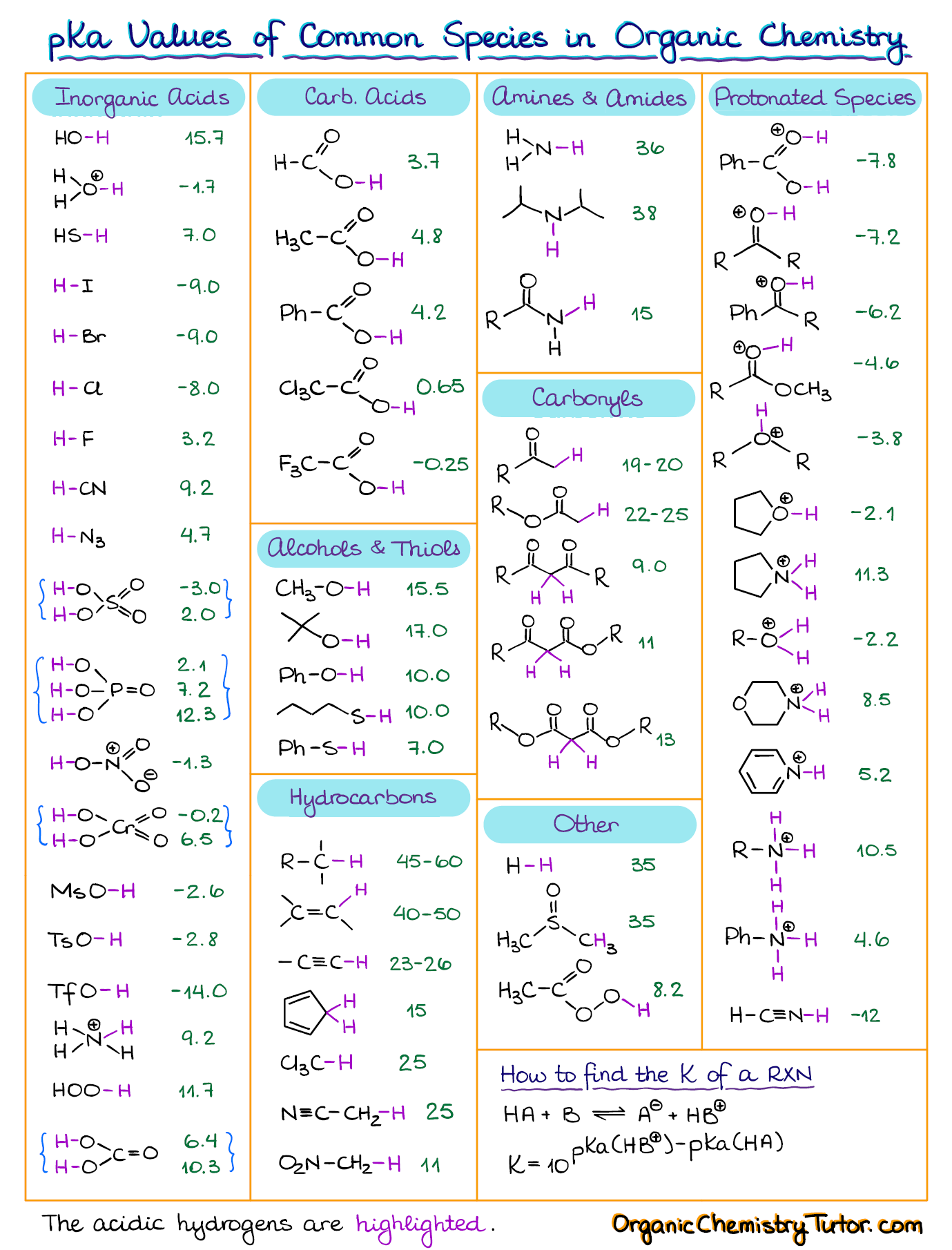
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic
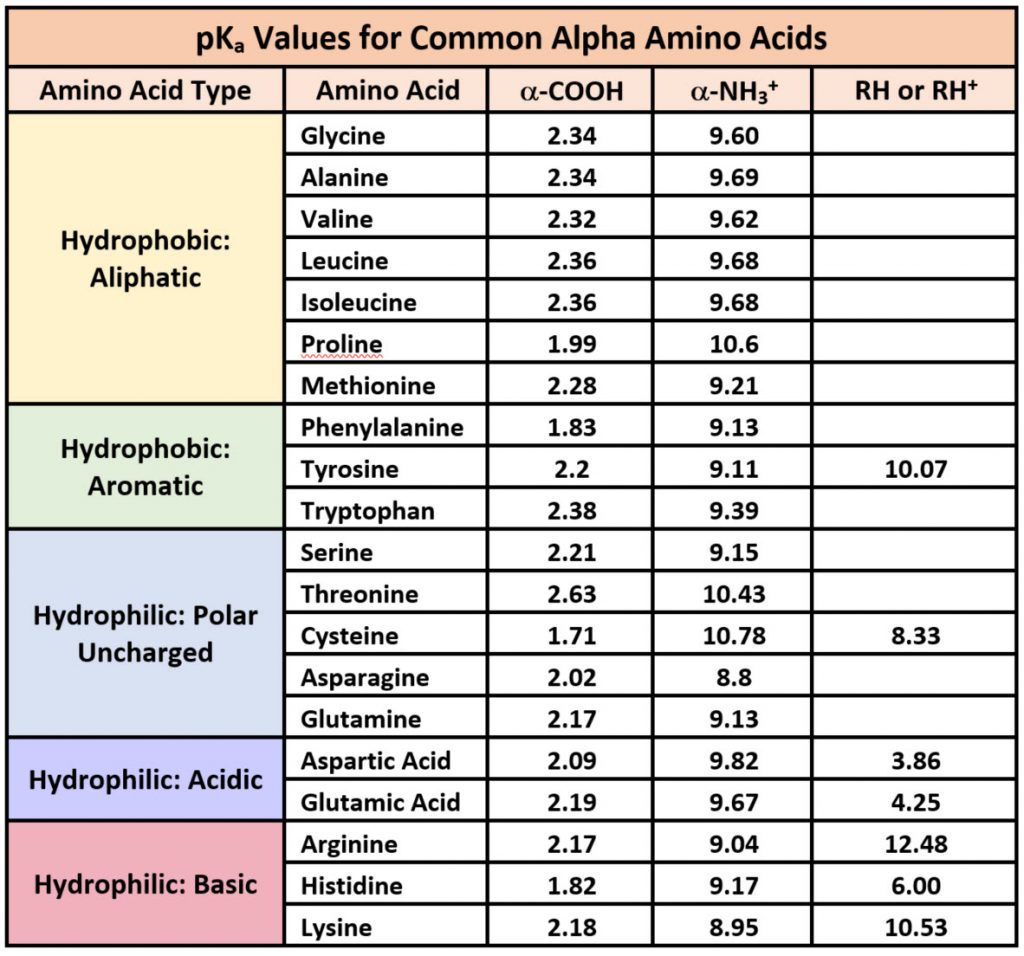
Chapter 2 Protein Structure Chemistry

Pka Chart Of Amino Acids
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial
Pka Chart Amino Acids

The pKa in Organic Chemistry Chemistry Steps
![[Infographic] Comprehensive pKa Chart r/chemistry](https://external-preview.redd.it/K3Snfd3HbLKkQbUsJ8g5GMVBN8te4Altg0_bder8QLE.jpg?auto=webp&s=d6f9b865541ac9cf9c578f5146e380a014396caa)
[Infographic] Comprehensive pKa Chart r/chemistry
Web Most Biochemistry Courses Will Require You To Know The Following:
Conjugate Acid Of − Nh 2, I.e., − Nh + 3 Has Pk A ~10.
Discover Our Full Product Line Of Amino Acids, Including Alanine, Isoleucine, Leucine, Valine, Phenylalanine, Tryptophan, Tyrosine, Aspargine, Cysteine, Glutamine, Methionine, Serine, Threonine, Aspartic Acid.
At Neutral Ph The Amino Group Is Protonated, And The Carboxyl Group Is Deprotonated.
Related Post: