Periodic Chart With Oxidation Numbers
Periodic Chart With Oxidation Numbers - First previous 1 next last. The oxidation number +3 is common to all lanthanides and actinides in their compounds. It has a valence of 1. Web each element cell contains the atomic number, symbol, name, atomic mass and most common valence charge or oxidation state of each element. Web what is oxidation number or oxidation state. The oxidation number represents how many electrons an atom has gained or lost in a molecule. The oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. The oxidation number represents the charge of an element in the compound. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. Web determine what is the oxidizing and reducing agents in the following reaction. Scandium is one of the two elements in the first transition metal period which has only one oxidation state (zinc is the other, with an oxidation state of +2). We can use oxidation numbers to keep track of where electrons are in a molecule, and how they move during a reaction. Web what is oxidation number or oxidation state. The. To keep track of electrons in a. Web charges given to atoms in a molecule in this way are called oxidation numbers. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! Web by assigning oxidation numbers to the atoms of each element in a. Learn how to determine oxidation numbers, along with examples, a diagram, and a chart. Web charges given to atoms in a molecule in this way are called oxidation numbers. Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to an element (a negative oxidation state) to get to. Rules for assigning oxidation numbers: We can use oxidation numbers to keep track of where electrons are in a molecule, and how they move during a reaction. Elements have an oxidation state of zero, and atoms in ionic compounds are usually assigned a. Web this color periodic table contains the number, symbol, name, atomic mass and oxidation states of each. We can use oxidation numbers to keep track of where electrons are in a molecule, and how they move during a reaction. The oxidation number represents the charge of an element in the compound. Web the following chart describes the most common oxidation states of the period 3 elements. It has a valence of 1. Important facts of oxidation numbers. Zn + 2h+ zn2+ +h2 zn + 2 h + zn 2 + + h 2. The oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. Visualize trends, 3d orbitals, isotopes, and mix compounds. Redox reactions are characterized by a transfer of electrons. Web this color periodic table. The oxidation number is also called as the oxidation state. A lithium atom has one outer shell electron. Information of oxidation numbers of monoatomic ions in periodic table. The oxidation number +3 is common to all lanthanides and actinides in their compounds. For best results, choose landscape and ‘fit’ for the size option. Elements have an oxidation state of zero, and atoms in ionic compounds are usually assigned a. Important facts of oxidation numbers. Zn + 2h+ zn2+ +h2 zn + 2 h + zn 2 + + h 2. It has a valence of 1. Reduction involves a decrease in oxidation state. Web periodic table with oxidation numbers. Web determine what is the oxidizing and reducing agents in the following reaction. What is the oxidation number when an element has not combined or do not form a compound. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. In this video,. Web periodic table with oxidation numbers. In this video, we'll use this method to identify the oxidized and reduced elements in the reaction that occurs between i⁻ and mno₄⁻ in basic solution. Web table of oxidation states of the elements. The oxidation number +3 is common to all lanthanides and actinides in their compounds. For best results, choose landscape and. Web each element cell contains the atomic number, symbol, name, atomic mass and most common valence charge or oxidation state of each element. Learn how to determine oxidation numbers, along with examples, a diagram, and a chart. Web this color periodic table contains the number, symbol, name, atomic mass and oxidation states of each element. Web the oxidation number is the positive or negative number of an atom that indicates the electrical charge the atom has if its compound consists of ions. More than one oxidation numbers of an element. In this video, we'll use this method to identify the oxidized and reduced elements in the reaction that occurs between i⁻ and mno₄⁻ in basic solution. The oxidation number is also called as the oxidation state. For best results, choose landscape and ‘fit’ for the size option. Zn + 2h+ zn2+ +h2 zn + 2 h + zn 2 + + h 2. It has a valence of 1. The oxidation number represents the charge of an element in the compound. The table is available for download in pdf format for offline printing. Information of oxidation numbers of monoatomic ions in periodic table. As the table shows, the presence of the other oxidation states varies, but follows some patterns. Web this periodic table contains the oxidation numbers of the elements as well as element numbers, symbols, names, and atomic weights. Redox reactions are characterized by a transfer of electrons./PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)
Periodic Table of the Elements Oxidation Numbers

Printable Periodic Table With Oxidation Numbers Two Birds Home
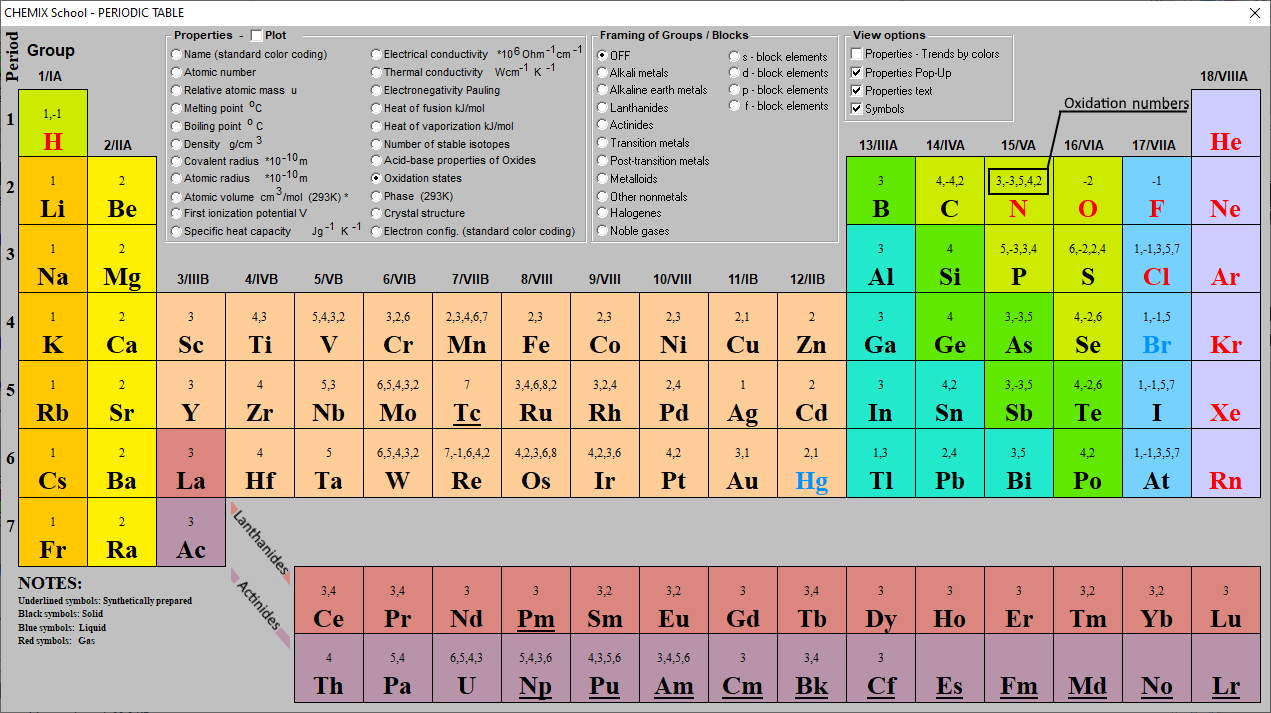
Periodic Table Of Oxidation Numbers Gordon Fastir
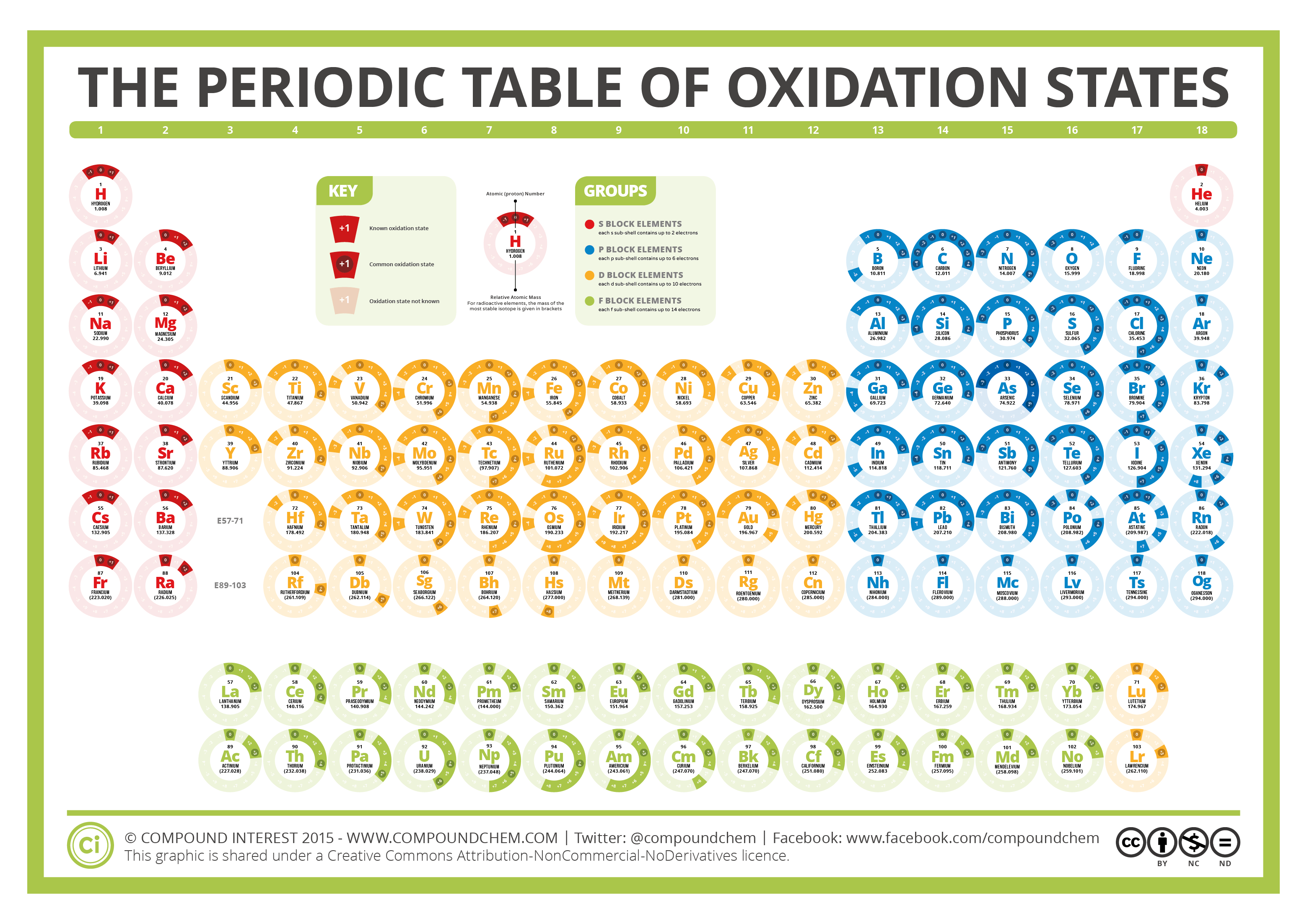
The Periodic Table of Oxidation States Compound Interest
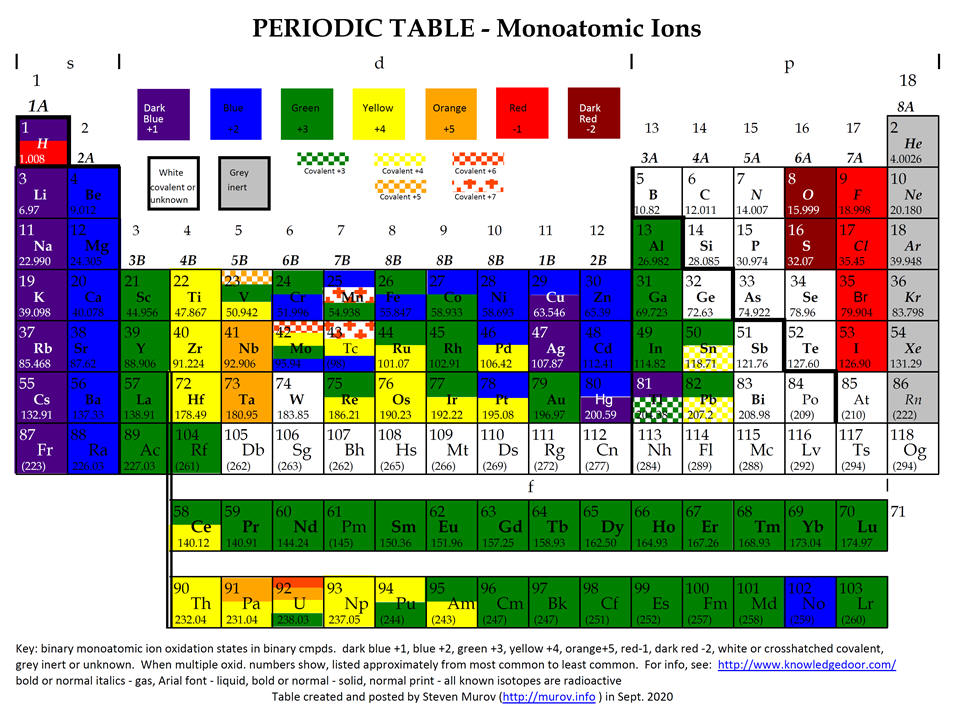
Chemistry Insight

periodic table oxidation Numbers Diagram Quizlet

Free Printable Periodic Tables (PDF and PNG) Science Notes and Projects
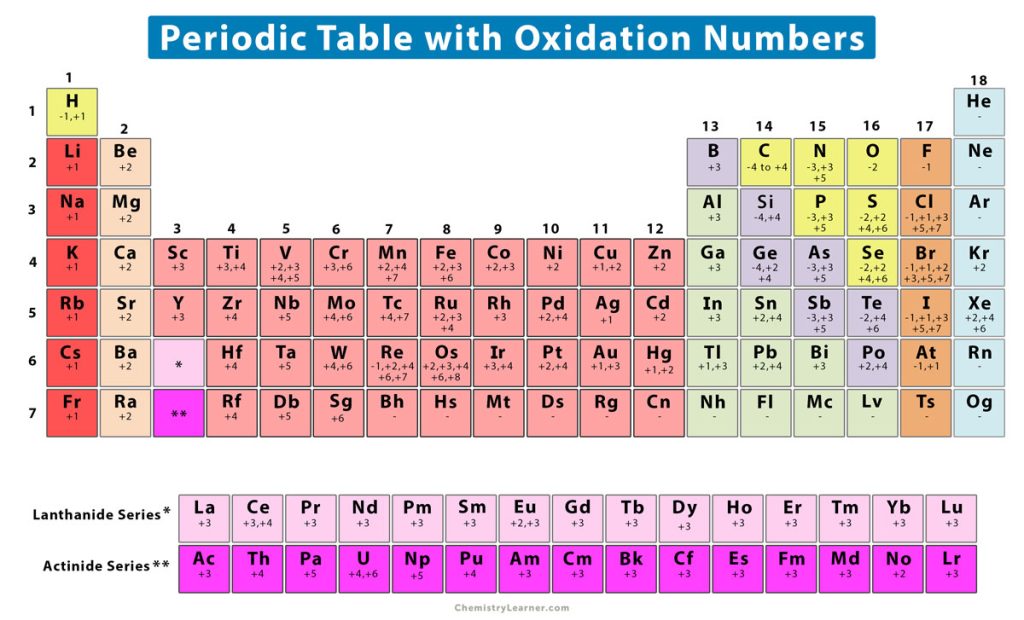
Oxidation Number (State) Definition, Rules, How to Find, and Examples
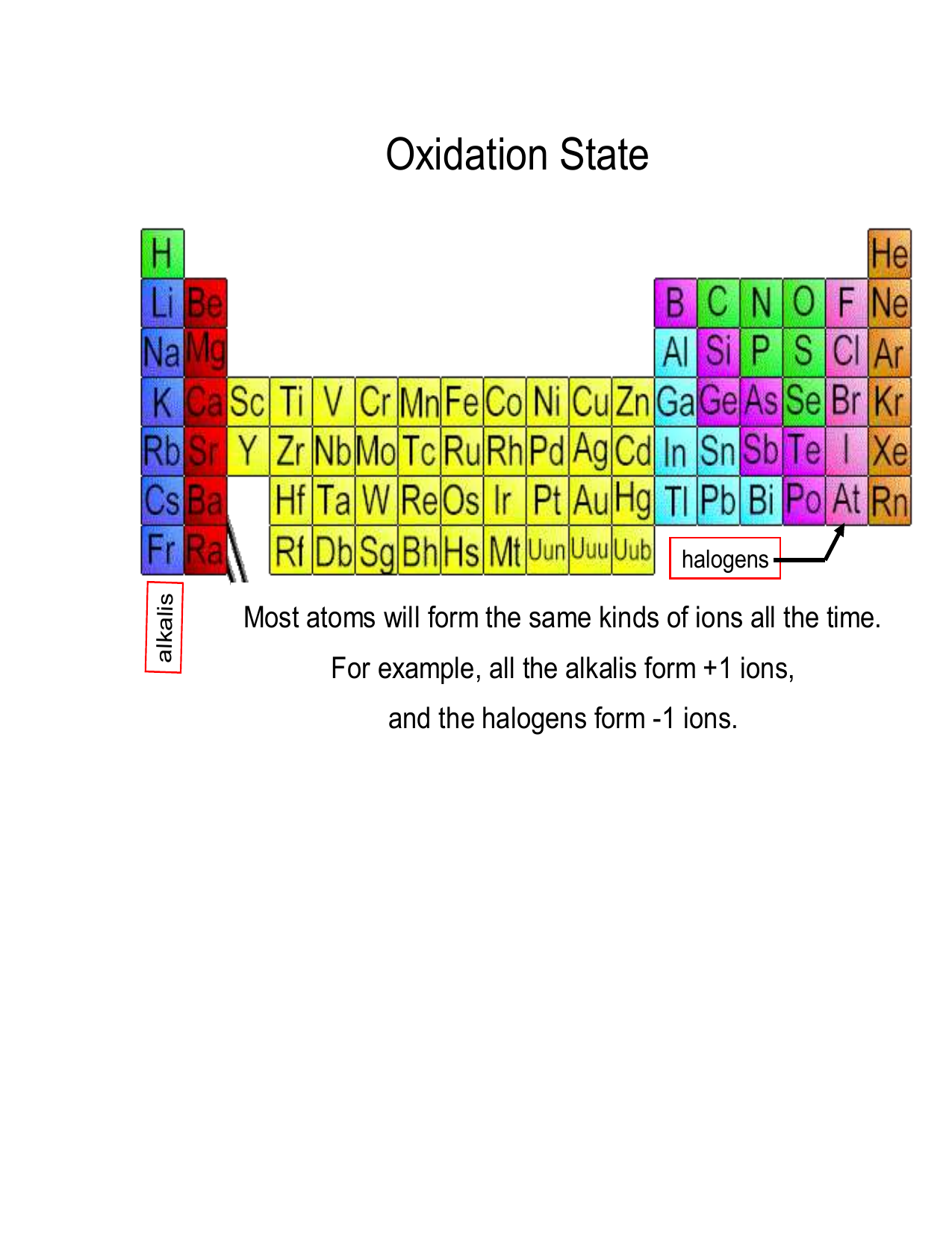
Oxidation Numbers

Downloadable Periodic Table Oxidation States
Oxidation Number Of An Atom When An Element Has Combined With The Same Element.
Web Table Of Oxidation States Of The Elements.
Web By Assigning Oxidation Numbers To The Atoms Of Each Element In A Redox Equation, We Can Determine Which Element Is Oxidized And Which Element Is Reduced During The Reaction.
We Can Use Oxidation Numbers To Keep Track Of Where Electrons Are In A Molecule, And How They Move During A Reaction.
Related Post: