Periodic Chart Of Ions
Periodic Chart Of Ions - Ions are single charged atoms (simple ions) or small charged “molecules” (polyatomic ions).”. Web ions are atoms with a charge. How to find ionic radius. For a single atom, the charge is the number of protons minus the number of electrons. Web you can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Web if the lithium atom becomes an ion, which type of ion will it be, a cation or an anion? What symbol will be used to represent it? Web for ions that do not form an isoelectronic series, locate their positions in the periodic table. Web here are two charts. The charge can be positive or negative. It is an icon of chemistry and is widely used in physics and other sciences. What will the ion be named? Web you can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. The diagram below shows the common charge of ions. Visualize trends, 3d orbitals, isotopes, and mix compounds. Web you can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. What symbol will be used to represent it? Web table of polyatomic ions. Learn what polyatomic ions are and how to write. What will the ion be named? As with simple ionic compounds, these compounds must also be electrically neutral, so their formulas can be predicted by treating the polyatomic ions as discrete units. Some ions consist of a single atom with a net charge. Web for ions that do not form an isoelectronic series, locate their positions in the periodic table.. See here for sal's explanation: Web interactive periodic table showing names, electrons, and oxidation states. When they do, they become monatomic ions. It indicates the size of an ion in a crystal lattice where two atoms are bonded by an ionic bond. Web for ions that do not form an isoelectronic series, locate their positions in the periodic table. Web for ions that do not form an isoelectronic series, locate their positions in the periodic table. The ionic radius is the distance of the outermost shell of electrons from the nucleus of an ion. Web 10 the periodic table of common ionic charges. What will the ion be named? Web here are two charts. To illustrate, an atom of an alkali metal (group 1. Other ions consist of a group of atoms with a net charge. See here for sal's explanation: Ions are single charged atoms (simple ions) or small charged “molecules” (polyatomic ions).”. In other words, the atom gains or loses an electron. Some ions consist of a single atom with a net charge. What will be the electric charge of this ion? Web here are two charts. Determine the relative sizes of the ions based on their principal quantum numbers n and their locations within a row. See here for sal's explanation: Examples include na + , o 2 − , and cl −. The charge can be positive or negative. Elements for which no ion is shown are those which do not commonly form ions, or superheavy elements whose chemistry is currently unknown. Common charges of ions formed by elements in. When they do, they become monatomic ions. How to find ionic radius. For a single atom, the charge is the number of protons minus the number of electrons. Web what is ionic radius. Web individual atoms can gain or lose electrons. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! Visualize trends, 3d orbitals, isotopes, and mix compounds. Web here are two charts. Web use this printable periodic table with element charges to predict compounds, oxidation states, and chemical reactions. Examples include na + , o 2 − , and cl −. Web table of polyatomic ions. It is an icon of chemistry and is widely used in physics and other sciences. Learn what polyatomic ions are and how to write them in chemical formulas. The proton never changes (if the proton changes, it is a different element)! Web 10 the periodic table of common ionic charges. Web here are two charts. What symbol will be used to represent it? For a single atom, the charge is the number of protons minus the number of electrons. Other ions consist of a group of atoms with a net charge. When atoms gain or lose electrons, they usually gain or lose a characteristic number of electrons and so take on a characteristic overall charge. Some ions consist of a single atom with a net charge. To illustrate, an atom of an alkali metal (group 1. See here for sal's explanation: Elements for which no ion is shown are those which do not commonly form ions, or superheavy elements whose chemistry is currently unknown. The ionic radius is the distance of the outermost shell of electrons from the nucleus of an ion. Web polyatomic ions are a group of bonded atoms that act as discrete units, carrying an overall charge. What will be the electric charge of this ion?/PeriodicTableCharge-WBG-56a12db23df78cf772682c37.png)
Periodic Table With Common Ionic Charges
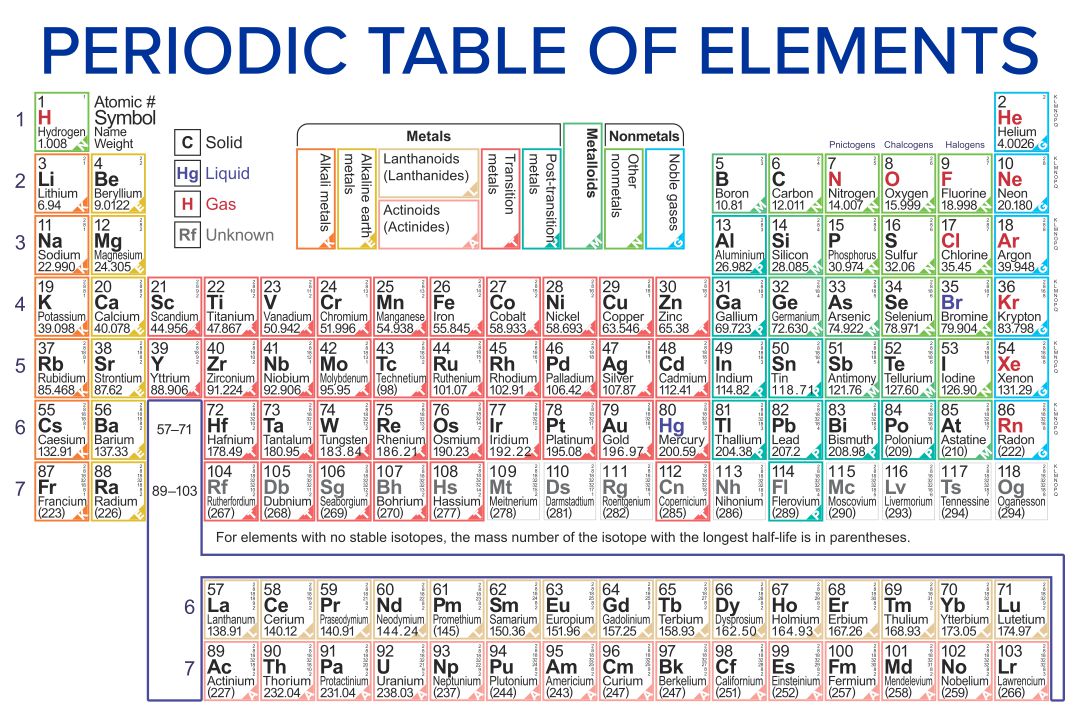
10 Best Printable Periodic Table Of Ions PDF for Free at Printablee
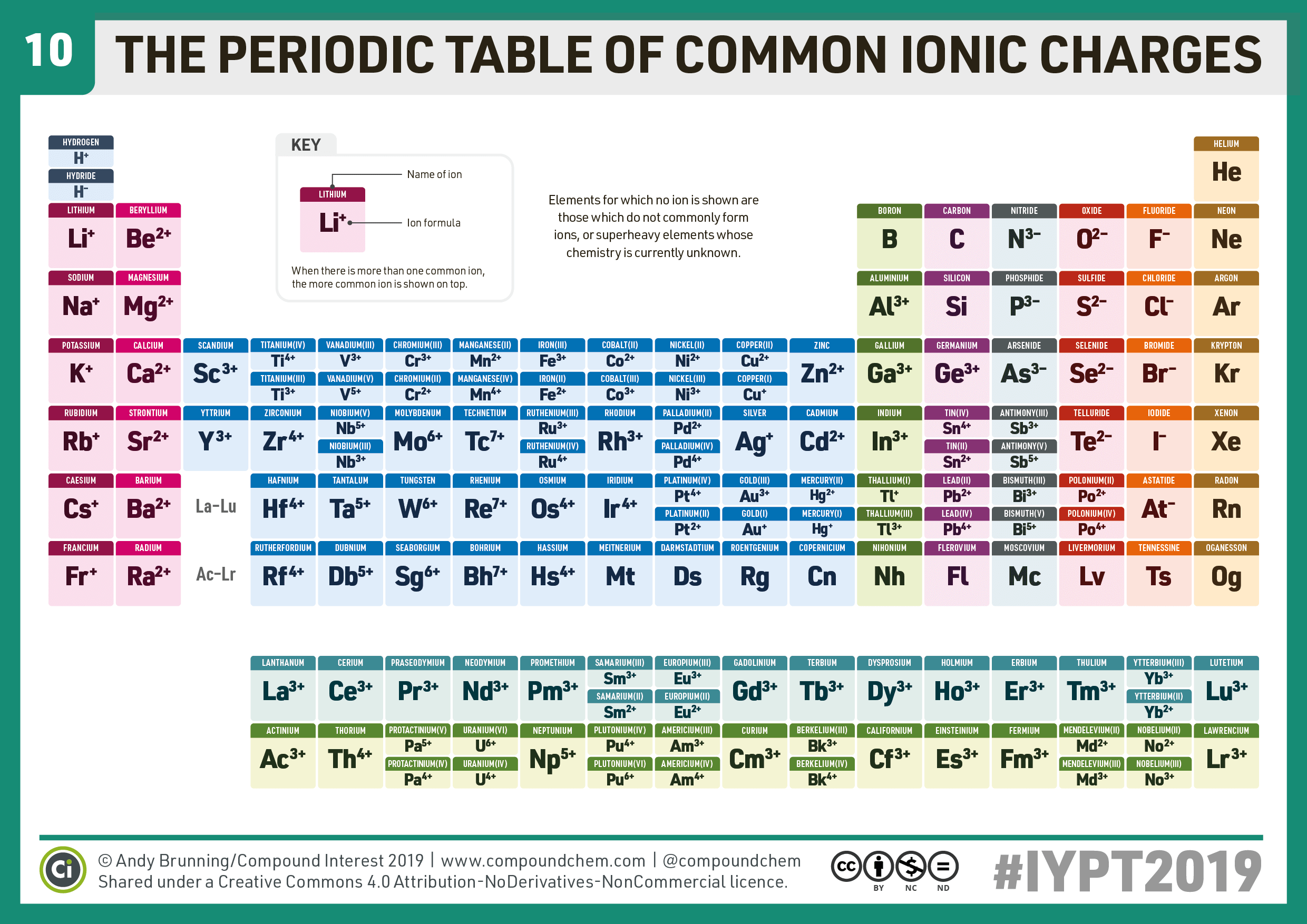
Compound Interest 10 Periodic Table of Common Ions
:max_bytes(150000):strip_icc()/PeriodicTableCharge-WBG-56a12db23df78cf772682c37.png)
Periodic Table With Common Ionic Charges
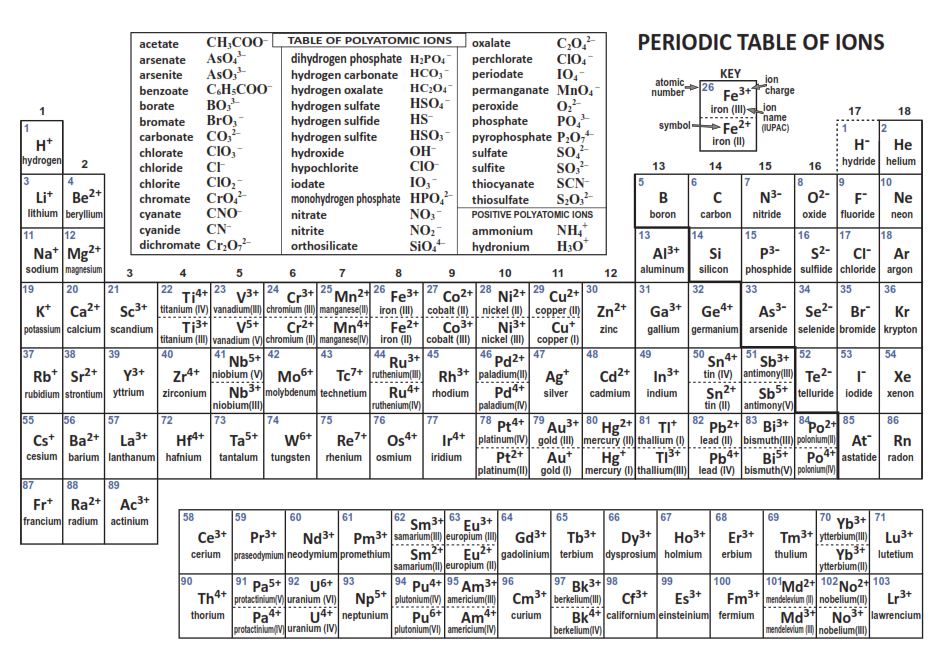
periodic table with ions Chemistry

Chem Ions Scientific Tutor

Compound Interest ChemistryAdvent IYPT2019 Day 10 A periodic table
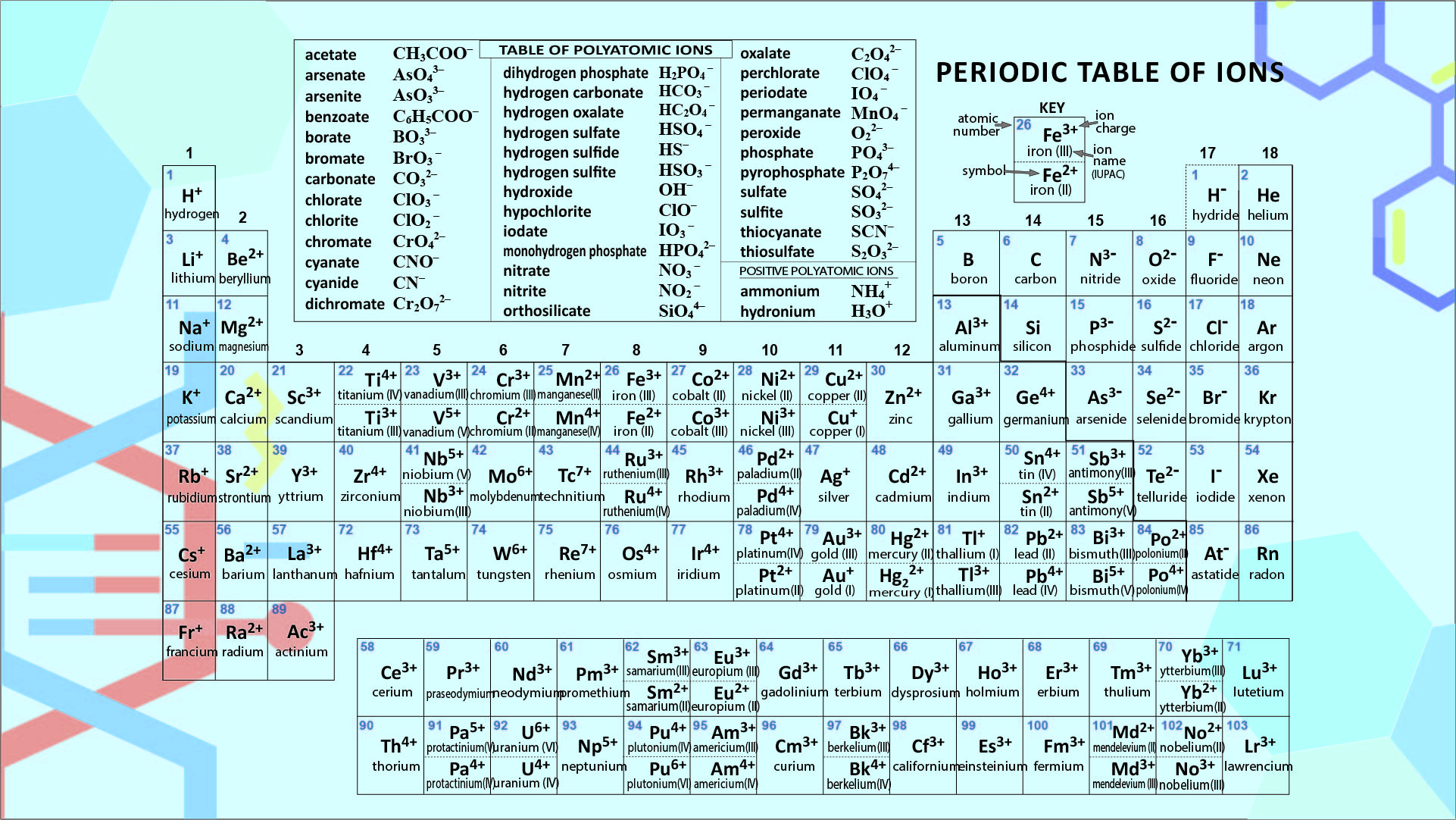
Periodic Table Of Ions 10 Free PDF Printables Printablee
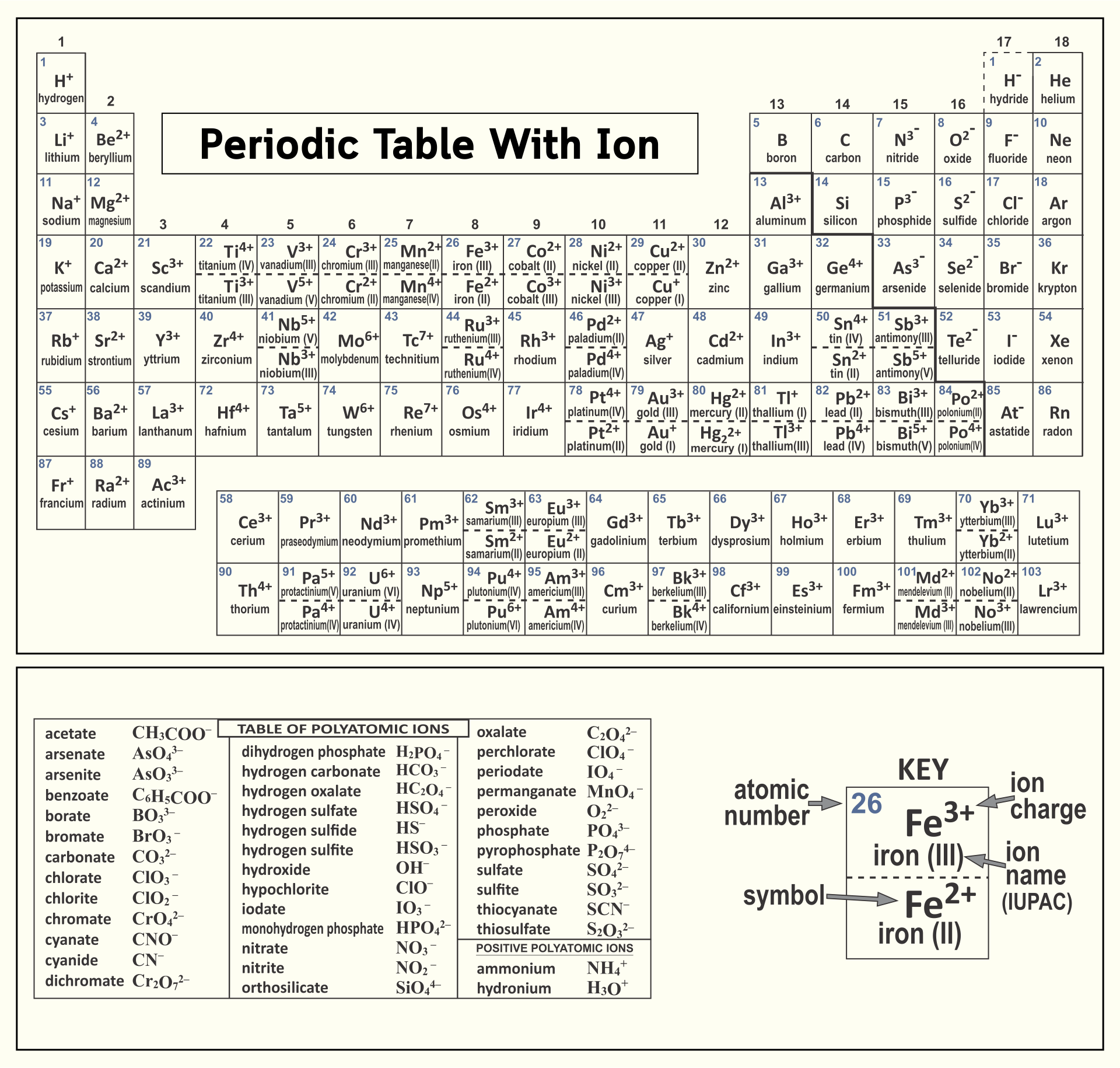
Periodic Table Ions List Periodic Table Timeline
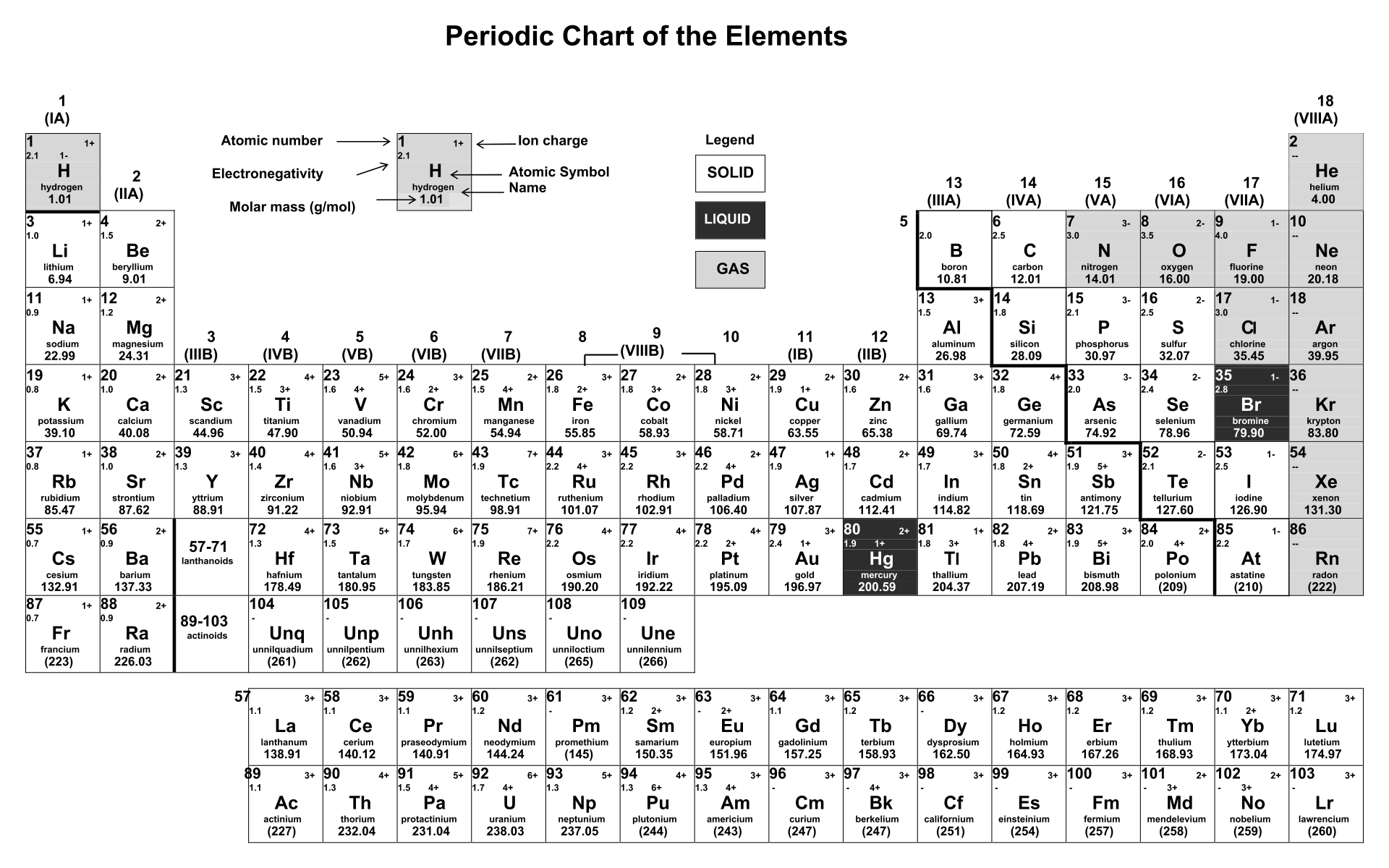
Periodic Table Of Ions 10 Free PDF Printables Printablee
Web The Periodic Table Is An Arrangment Of The Chemical Elements Ordered By Atomic Number So That Periodic Properties Of The Elements (Chemical Periodicity) Are Made Clear.
As With Simple Ionic Compounds, These Compounds Must Also Be Electrically Neutral, So Their Formulas Can Be Predicted By Treating The Polyatomic Ions As Discrete Units.
Determine The Relative Sizes Of The Ions Based On Their Principal Quantum Numbers N And Their Locations Within A Row.
Web For Ions That Do Not Form An Isoelectronic Series, Locate Their Positions In The Periodic Table.
Related Post: