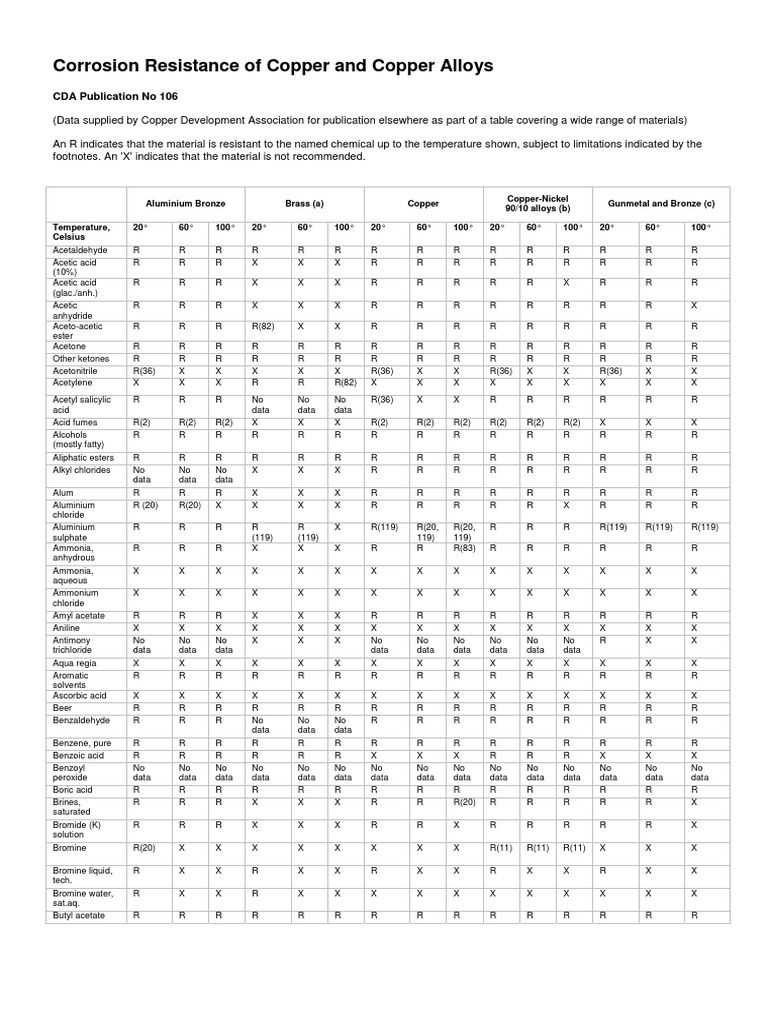
Metal To Metal Corrosion Chart
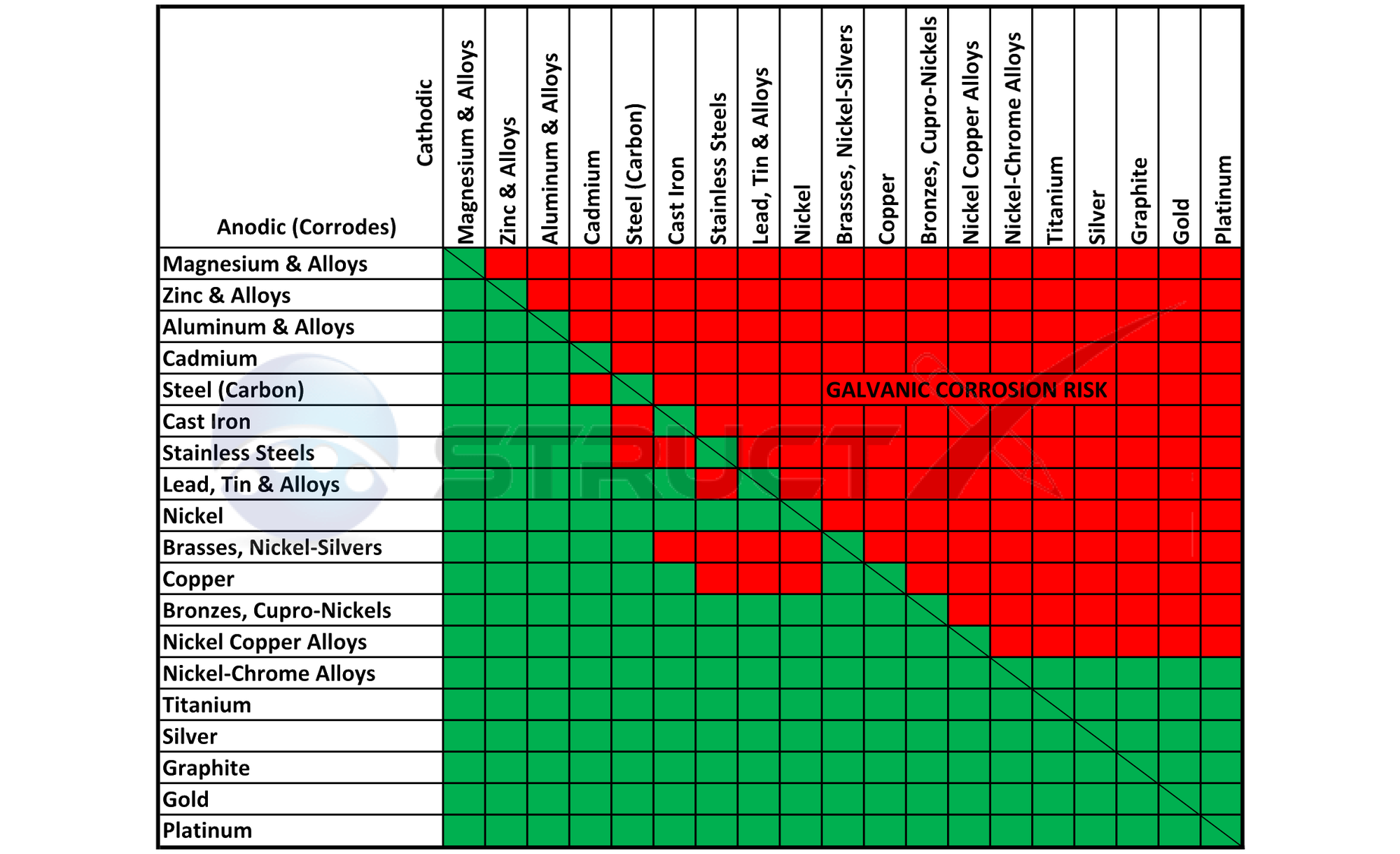
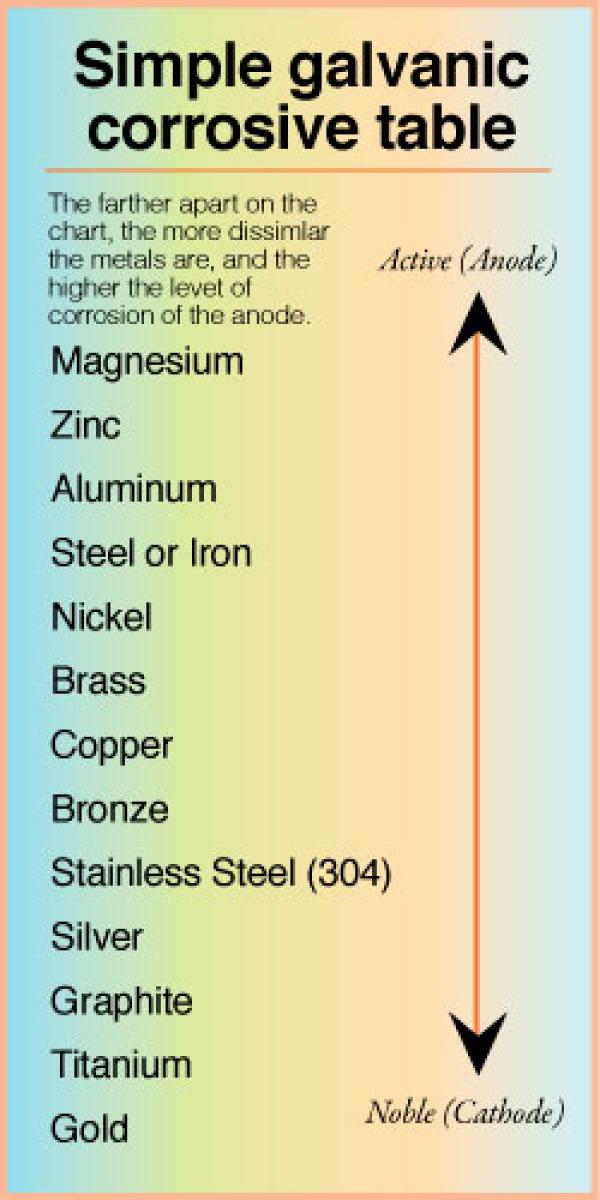
Metal To Metal Corrosion Chart - Web what exactly is the galvanic series? Web common materials include neoprene, rubber, plastic, mylar, nylon, teflon, glass reinforced epoxy (gre) gaskets, and more. Web corrosion theory for metals. Web below is a galvanic reaction chart for dissimilar metals. The ‘cell’ produced can result in corrosion to one of the paired metals. What is the galvanic series? Web as the series suggests, steel and aluminum are relatively compatible, but if brass and steel contact, the steel will corrode because it is more anodic than the brass. (noble metals are those that are resistant to corrosion and oxidation.) This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Web below is a galvanic reaction chart for dissimilar metals. Web what is galvanic corrosion: Web this chart will help you to determine which metals are more noble than other metals. Align the metal to be assessed for the risk of corrosion in the rows (coordinating metal) with the contact metal (columns). Web what is galvanic corrosion? We also provide other helpful methods for avoiding galvanic corrosion. Corrosion is defined as an attack on a material as a result of chemical, frequently electrochemical reaction, with the surrounding medium. Align the metal to be assessed for the risk of corrosion in the rows (coordinating metal) with the contact metal (columns). Web what is galvanic corrosion: The ‘cell’ produced can result in corrosion to one of the paired metals.. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: Contact a corrosion specialist to determine the best material for your application. What are the causes of galvanic corrosion? We also provide other helpful methods for avoiding galvanic corrosion. Bimetallic corrosion can only occur when two dissimilar metals are in ‘electrical’ contact. Types of galvanic corrosion in different metals and their alloys. Corrosion is defined as an attack on a material as a result of chemical, frequently electrochemical reaction, with the surrounding medium. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: In this article, we will discuss what is galvanic corrosion, its. Please understand that green represents lower risk not no risk. it should be noted that if sacrificial plating is incorporated in the fastener design, then galvanic action can result in the deterioration of the sacrificial coating, rather than of the fastener. Types of galvanic corrosion in different metals and their alloys. Corrosion of metals in soil is extremely variable and. Web common materials include neoprene, rubber, plastic, mylar, nylon, teflon, glass reinforced epoxy (gre) gaskets, and more. Corrosion criteria based on laboratory tests are commonly expressed in grams per square meter per hour. Web bimetallic (galvanic) corrosion risks from contact with galvanised steel or aluminium. (noble metals are those that are resistant to corrosion and oxidation.) Web this chart is. Web bimetallic (galvanic) corrosion risks from contact with galvanised steel or aluminium. If brass and aluminum plates are connected by a passivated 304. Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Web what. Web bimetallic (galvanic) corrosion risks from contact with galvanised steel or aluminium. Corrosion of metals in soil is extremely variable and while the soil environment is complex, it is possible to make some generalizations about soil types and corrosion. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other. (noble metals are those that are resistant to corrosion and oxidation.) Web this chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Rivets, bolts, spot welds sustained cathodic reaction: What is the galvanic series? Web below, we give a brief overview of galvanic corrosion and. Corrosion of metals in soil is extremely variable and while the soil environment is complex, it is possible to make some generalizations about soil types and corrosion. Web below is a galvanic reaction chart for dissimilar metals. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web common metals and their corrosion resistance to aggressive fluids like acids, bases and more. What is the galvanic series? Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal combinations. Web here we explain the galvanic scale, the effect of corrosion caused when certain metals are placed in contact, and we provide examples of galvanic corrosion hazards that occur in buildings metal roofing, building electrical components, building plumbing components, and at underground oil storage tanks and oil piping systems. Web as the series suggests, steel and aluminum are relatively compatible, but if brass and steel contact, the steel will corrode because it is more anodic than the brass. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Web corrosion theory for metals. Corrosion is defined as an attack on a material as a result of chemical, frequently electrochemical reaction, with the surrounding medium. Web below is a galvanic reaction chart for dissimilar metals. Web one exception to the necessity for the two metals or alloys to be in direct electrical contact for metallic corrosion to occur, is when the noble metal corrodes slightly and dissolves in water which subsequently flows over a less noble material. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. We also provide other helpful methods for avoiding galvanic corrosion. Types of galvanic corrosion in different metals and their alloys. (noble metals are those that are resistant to corrosion and oxidation.)Corrosion Resistance Chart (Copper and Copper Alloy).pdf Brass

Galvanic Corrosion Chart Dissimilar Metals Video Bokep Ngentot
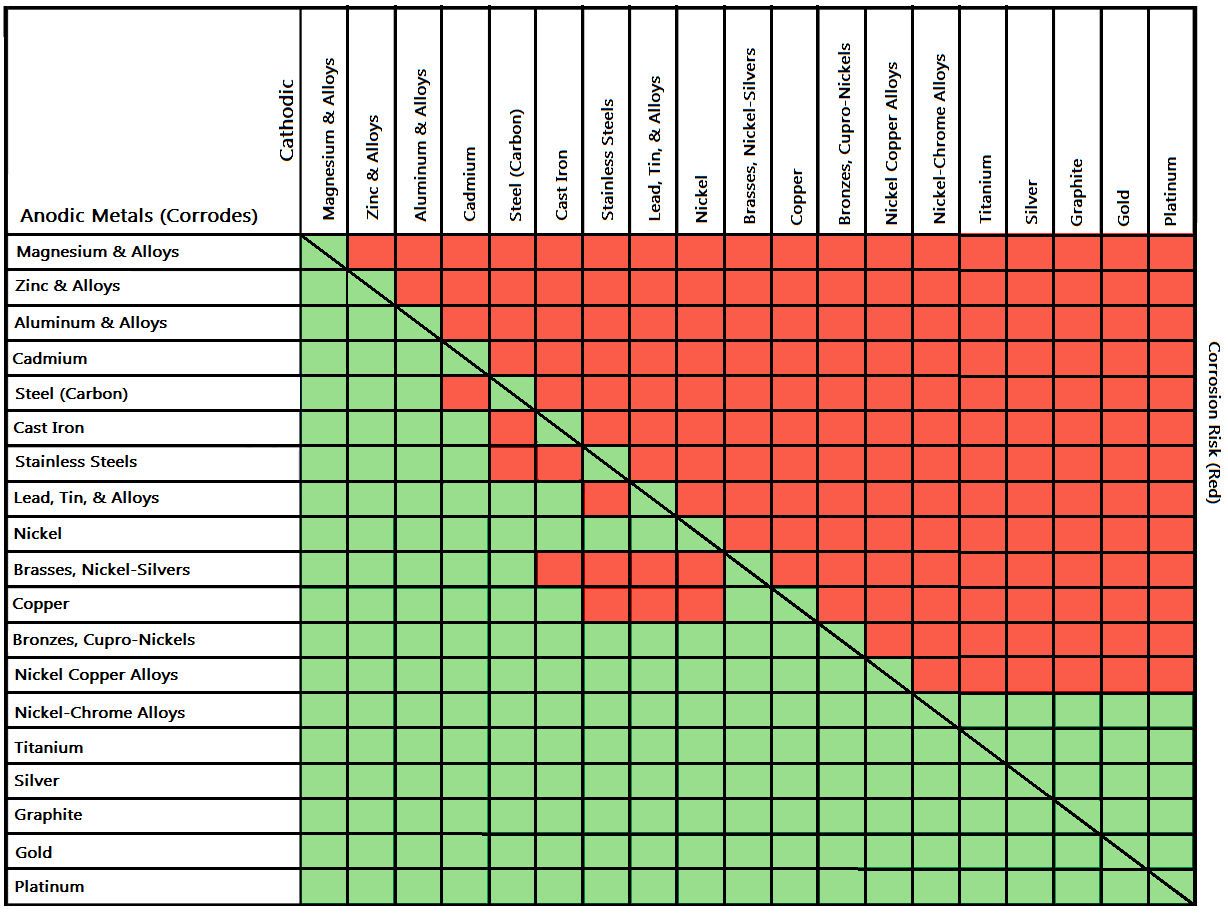
Galvanic Corrosion Common Questions Answered
Galvanic Corrosion PDF Corrosion Stainless Steel
![Galvanic Corrosion [with Chart] EngineerExcel](https://engineerexcel.com/wp-content/uploads/2023/03/galvanic-corrosion-chart.webp)
Galvanic Corrosion [with Chart] EngineerExcel
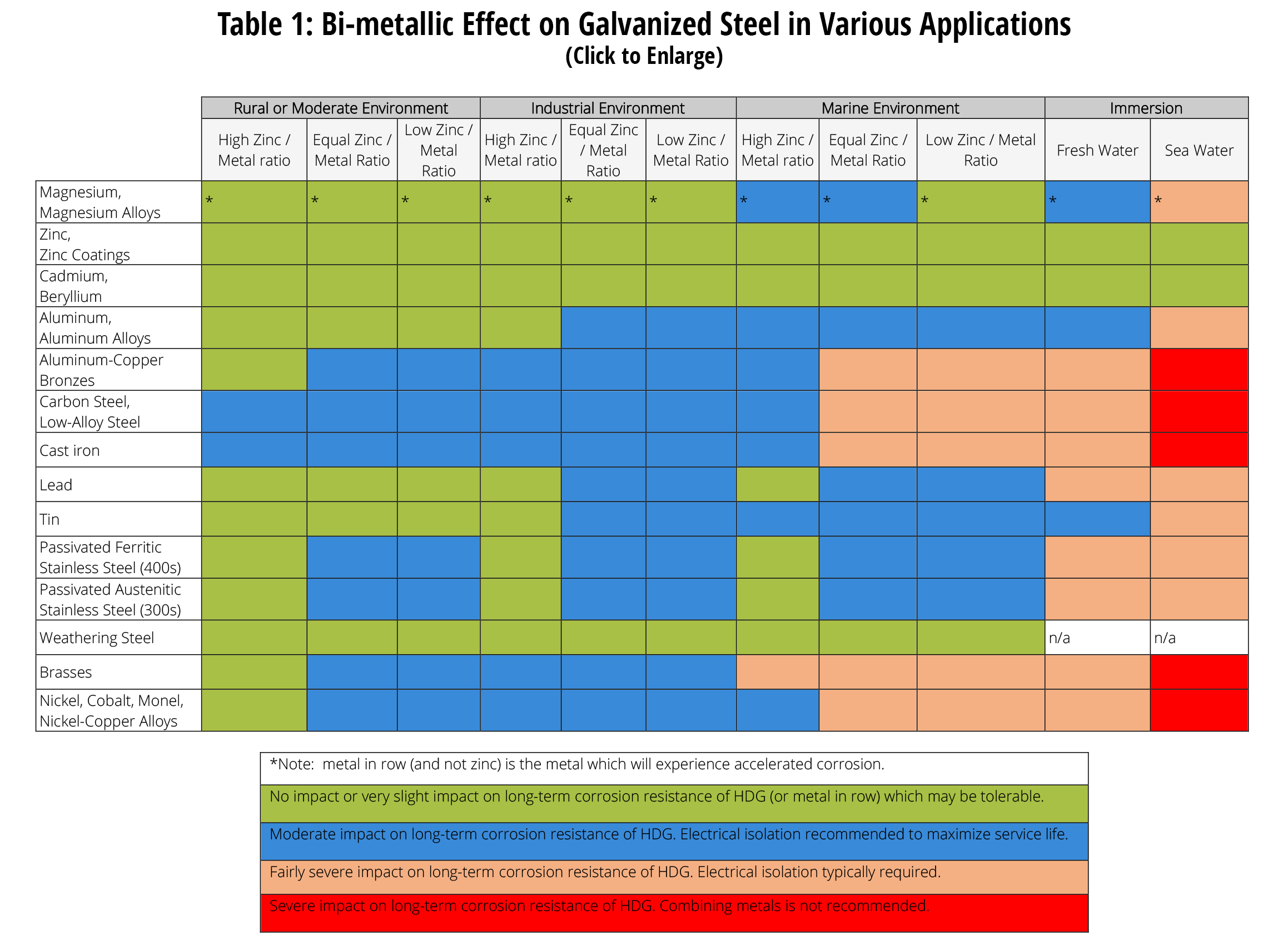
Stainless Steel Galvanic Corrosion Chart
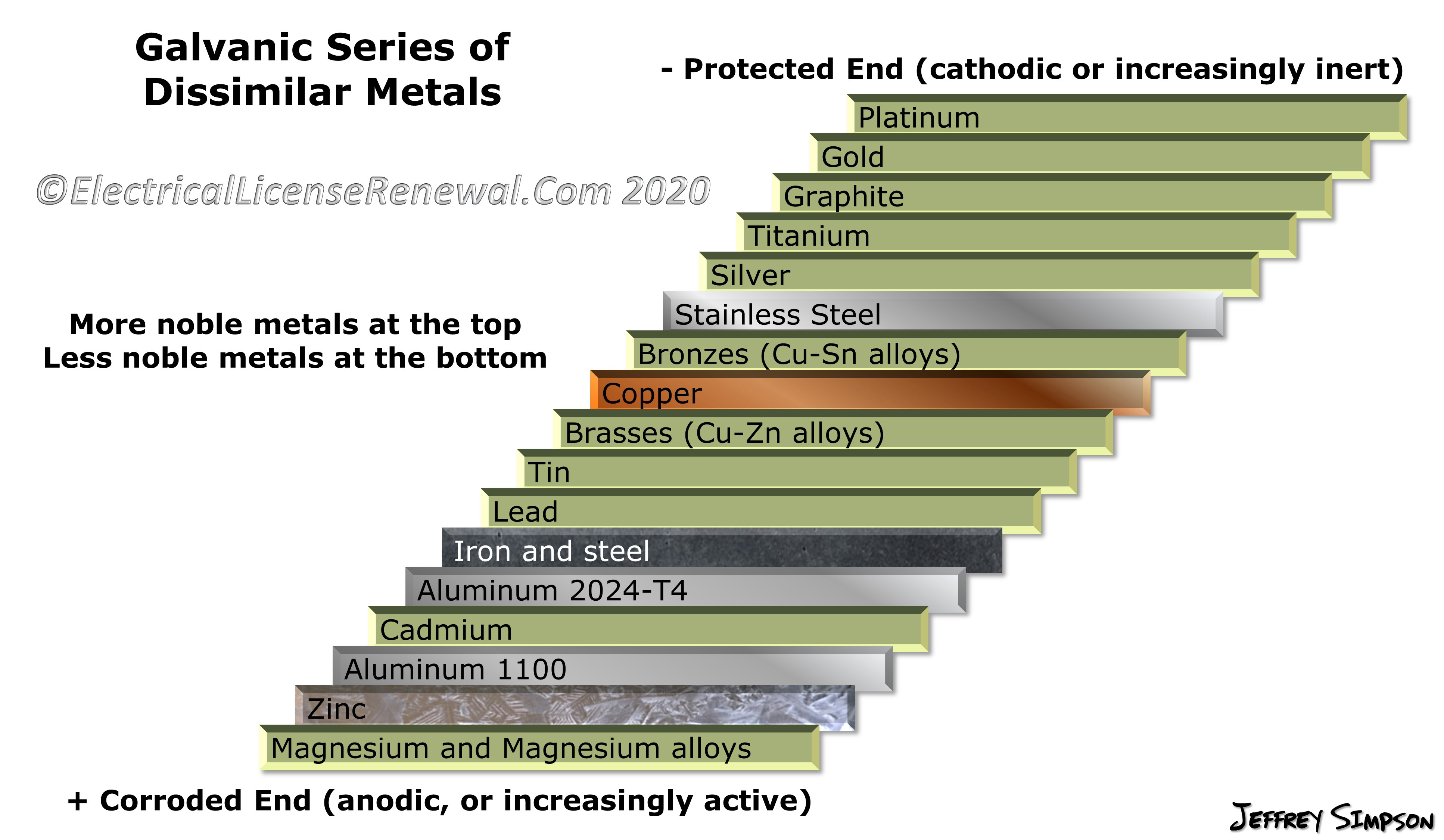
Stainless Steel Galvanic Corrosion Chart
Aluminum Corrosion Resistance Chart

Galvanic Corrosion Chart Metals
Stainless Steel Galvanic Corrosion Chart
Corrosion Of Metals In Soil Is Extremely Variable And While The Soil Environment Is Complex, It Is Possible To Make Some Generalizations About Soil Types And Corrosion.
If Brass And Aluminum Plates Are Connected By A Passivated 304.
The ‘Cell’ Produced Can Result In Corrosion To One Of The Paired Metals.
Please Understand That Green Represents Lower Risk Not No Risk. It Should Be Noted That If Sacrificial Plating Is Incorporated In The Fastener Design, Then Galvanic Action Can Result In The Deterioration Of The Sacrificial Coating, Rather Than Of The Fastener.
Related Post: