Glycol Water Mixture Chart
Glycol Water Mixture Chart - After placing a sample of the glycol in the beaker, check the reading on the hydrometer and match it to the appropriate chart to accurately determine the glycol to water weight percentage. Web the conversion charts below are to be used with the ppe precision specific gravity hydrometer and beaker. Web ethylene glycol heat transfer fluid freeze point chart freezing point °f freezing point °c boiling point °f/760 mm/hg boiling point °c@ 0.96 / barr Table obtained from lange's handbook of chemistry, 10th ed. The main job of glycol is to prevent freezing of the process fluid and ensure consistent flow at the operating temperature. Water freezes at 32° f; Ethylene and propylene are the two standard types of inhibited glycols commonly used. Web the use of an industrial inhibited glycol and water mixture is recommended in most water chiller systems. Web skip to glycol concentration chart. Web ethylene glycol as an antifreeze is based on its ability to lower the freezing point when mixed with water. Web ethylene glycol heat transfer fluid freeze point chart freezing point °f freezing point °c boiling point °f/760 mm/hg boiling point °c@ 0.96 / barr Table obtained from lange's handbook of chemistry, 10th ed. The highest yields of ethylene glycol occur at acidic or neutral ph with a large excess of water. Web skip to glycol concentration chart. Web glycol. ¤ propylene glycol ¤ inhibitor package & water ¤ color ¤ ph of solution @ 50% glycol ¤ specific gravity @ 60/60 f ¤ reserve alkalinity (min) 95% 5% colorless. Modified on 20 december 2017. After placing a sample of the glycol in the beaker, check the reading on the hydrometer and match it to the appropriate chart to accurately. (ii) adjusting the fit for the density of pure glycerine as a function of temperature. This reaction can be catalyzed by either acids or bases, or can occur at neutral ph under elevated temperatures. Web learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Table obtained from lange's handbook of chemistry, 10th ed. Web glycols. The charts are based on different concentrations at various temperatures. Web andreas volk pointed out that the density calculation can be made more accurate by (i) accounting for the volume contraction of the mixture; Automatic makeup water systems should be avoided to prevent undetected dilution or loss of glycol. Fill in all white input fields and click ‘calculate’. Web the. Web reference this chart in deciding how much glycol is necessary for your product. Web freezing point of aqueous solutions. Web the use of an industrial inhibited glycol and water mixture is recommended in most water chiller systems. Web ethylene glycol as an antifreeze is based on its ability to lower the freezing point when mixed with water. However, glycol. The main job of glycol is to prevent freezing of the process fluid and ensure consistent flow at the operating temperature. Calculate concentration for glycol freezing point or burst point protection. Web ethylene glycol heat transfer fluid freeze point chart freezing point °f freezing point °c boiling point °f/760 mm/hg boiling point °c@ 0.96 / barr In both cases the. Web the use of an industrial inhibited glycol and water mixture is recommended in most water chiller systems. Mixing the two lowers the freezing point of water, allowing it to run through a chiller system at. After placing a sample of the glycol in the beaker, check the reading on the hydrometer and match it to the appropriate chart to. The end uses for ethylene glycol are numerous (see table 1). Web comparing properties like specific gravity, freezing points and viscosity for secondary coolants like calcium chloride, sodium chloride, ethylene glycol and propylene glycol. Water freezes at 32° f; However, glycol freezes at 9° f. Web learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Web learn about the chemical and physical properties of ethylene glycol/water mixtures, on our blog. Web propylene glycol heat transfer fluid freeze point chart freezing point °f freezing point °c boiling point °f/760 mm/hg boiling point °c@ 0.96/barr propylene glycol wt. Properties differs so much from clean water that heat transfer systems with ethylene glycol should be calculated thoroughly for. Web comparing properties like specific gravity, freezing points and viscosity for secondary coolants like calcium chloride, sodium chloride, ethylene glycol and propylene glycol. The highest yields of ethylene glycol occur at acidic or neutral ph with a large excess of water. Thus, to achieve the same heat exchange inside the heat exchanger , requires more surface area or a larger. The main job of glycol is to prevent freezing of the process fluid and ensure consistent flow at the operating temperature. Web the conversion charts below are to be used with the ppe precision specific gravity hydrometer and beaker. Web ethylene glycol heat transfer fluid freeze point chart freezing point °f freezing point °c boiling point °f/760 mm/hg boiling point °c@ 0.96 / barr Specific gravity is referenced to water at 15.6 °c. Fill in all white input fields and click ‘calculate’. The end uses for ethylene glycol are numerous (see table 1). Do not mix ethylene and propylene glycols in the same system. Web freezing point of aqueous solutions. There are two basic protection points: Freeze protection and burst protection. Ethylene and propylene are the two standard types of inhibited glycols commonly used. Web see the charts below for help determining what percentage of glycol your system needs. However, glycol freezes at 9° f. After placing a sample of the glycol in the beaker, check the reading on the hydrometer and match it to the appropriate chart to accurately determine the glycol to water weight percentage. This reaction can be catalyzed by either acids or bases, or can occur at neutral ph under elevated temperatures. Web the use of an industrial inhibited glycol and water mixture is recommended in most water chiller systems.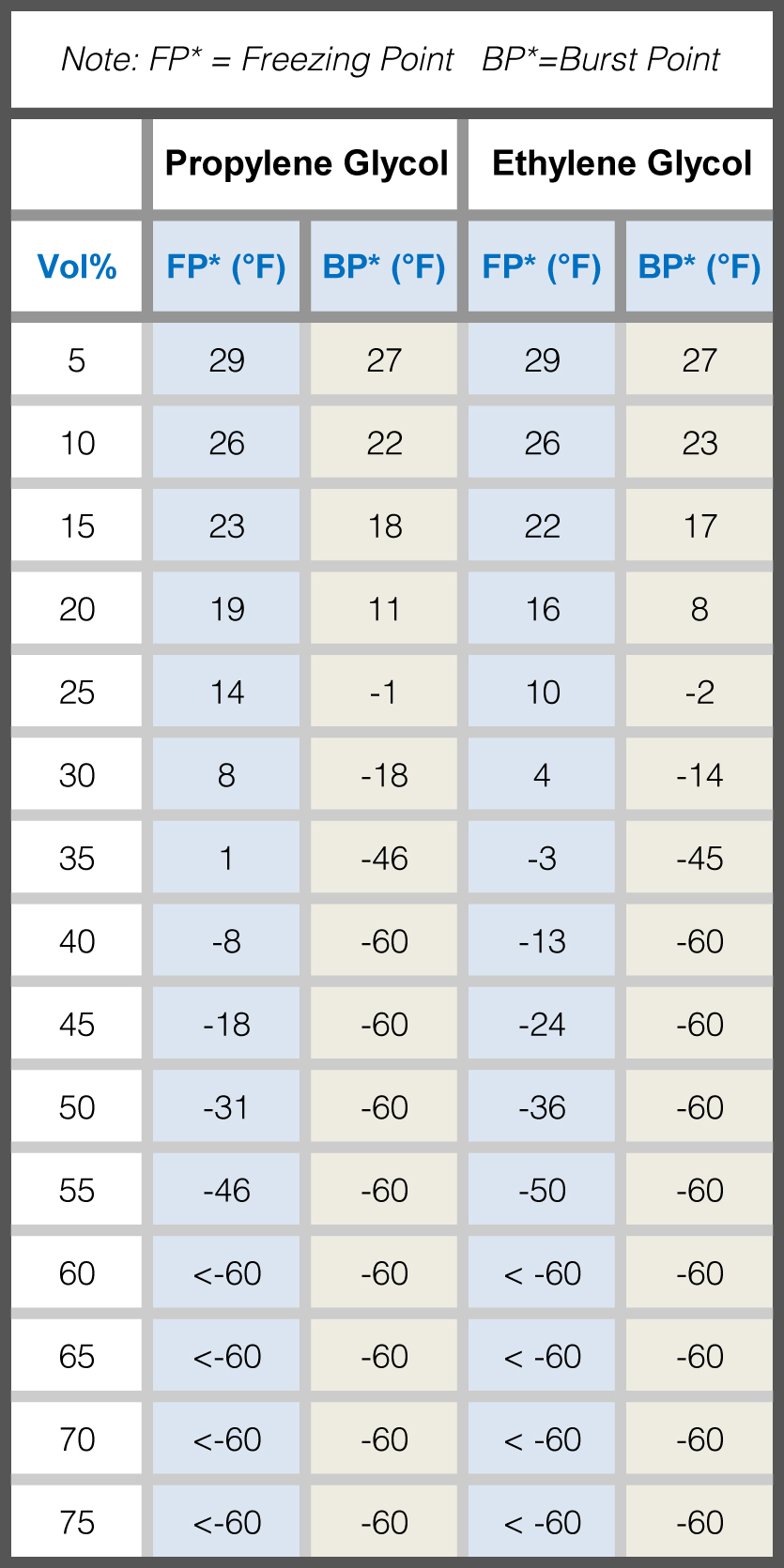
Ethylene Glycol Water Mixture Density Table
Ethylene Glycol Water Mixture Density Table

Ethylene Glycol Water Mixture Density Table

WaterGlycol mixture measurement Baker Hughes

Ethylene Glycol Water Mixture Density Table
![[PDF] Ethylene Glycol and Its Mixtures with Water and Electrolytes](https://d3i71xaburhd42.cloudfront.net/b802f6702e8678ea6b78b956f251743775579bf2/14-Figure15-1.png)
[PDF] Ethylene Glycol and Its Mixtures with Water and Electrolytes

Propylene Glycol To Water Ratio Chart

Ethylene Glycol Freeze Chart

Table 6 from Ethylene Glycol and Its Mixtures with Water and

Ethylene Glycol Temperature Chart A Visual Reference of Charts Chart
Web Glycols Are Heavy, Syrup Like Fluids At Full Concentration, And Become Thinner When Mixed With Water.
Web C2H4O + H2O → Ho−Ch2Ch2−Oh.
Water Freezes At 32° F;
Modified On 20 December 2017.
Related Post: