Glp 1 Agonist Conversion Chart
Glp 1 Agonist Conversion Chart - Due to gl adverse events 3.1% 3.1% 6% (vs. Researchers say this experimental drug increased. The prescribing information for semaglutide states that patients should be initiated at 0.25 mg per week for 4 weeks,. Some also have documented cardiovascular benefit. Dose reductions & other considerations. And some of their featured characteristics. Injections are given under the skin. Drugs are listed alphabetically when specific drugs are suggested, the order in the table is therefore not an order of preference. Ozempic (semaglutide) and victoza (liraglutide) are examples that treat type 2 diabetes. A weight reduction of ≥3% (of initial body weight) additional information about dosing and licensed combinations, precautions and special warning for use can be found at www.medicines.org.uk. Web published june 25, 2021. A weight reduction of ≥3% (of initial body weight) additional information about dosing and licensed combinations, precautions and special warning for use can be found at www.medicines.org.uk. Drugs are listed alphabetically when specific drugs are suggested, the order in the table is therefore not an order of preference. Some also have documented cardiovascular benefit. Summary. The researchers followed up the children of more than 50. Summary of clinical evidence and comparison chart adverse effects: Examples of drugs in this class include exenatide, liraglutide, dulaglutide, and semaglutide. Dulaglutide (trulicity®) exenatide (byetta®) exenatide (bydureon bcise®) ᴾ. This guide does not replace clinical judgment. The researchers followed up the children of more than 50. N engl j med 2021;385: Drugs are listed alphabetically when specific drugs are suggested, the order in the table is therefore not an order of preference. An oral option is also available. Some also have documented cardiovascular benefit. Web triggers for switching glp‐1ras, recommended glp‐1ras to switch to and the expected benefits of switching. And some of their featured characteristics. An oral option is also available. Drugs are listed alphabetically when specific drugs are suggested, the order in the table is therefore not an order of preference. N engl j med 2021;385: Ozempic (semaglutide) and victoza (liraglutide) are examples that treat type 2 diabetes. Web triggers for switching glp‐1ras, recommended glp‐1ras to switch to and the expected benefits of switching. In addition, an oral preparation of semaglutide (rybelsus®) has been licensed for use. An oral option is also available. Some also have documented cardiovascular benefit. (jama network open)in a phase ib trial. Drugs are listed alphabetically when specific drugs are suggested, the order in the table is therefore not an order of preference. Due to gl adverse events 3.1% 3.1% 6% (vs. And some of their featured characteristics. Dulaglutide (trulicity®) exenatide (byetta®) exenatide (bydureon bcise®) ᴾ. Injections are given under the skin. Researchers say this experimental drug increased. Ozempic (semaglutide) and victoza (liraglutide) are examples that treat type 2 diabetes. (jama network open)in a phase ib trial. In addition, an oral preparation of semaglutide (rybelsus®) has been licensed for use. Injections are given under the skin. Dose reductions & other considerations. N engl j med 2021;385: A weight reduction of ≥3% (of initial body weight) additional information about dosing and licensed combinations, precautions and special warning for use can be found at www.medicines.org.uk. Summary of clinical evidence and comparison chart adverse effects: Some are also approved for weight loss. Dulaglutide (trulicity®) exenatide (byetta®) exenatide (bydureon bcise®) ᴾ. Drugs are listed alphabetically when specific drugs are suggested, the order in the table is therefore not an order of preference. The researchers followed up the children of more than 50. Injections are given under the skin. In addition, an oral preparation of semaglutide (rybelsus®) has been licensed for use. The researchers followed up the children of more than 50. Dulaglutide (trulicity®) exenatide (byetta®) exenatide (bydureon bcise®) ᴾ. Researchers say this experimental drug increased. An oral option is also available. Injections are given under the skin. Web triggers for switching glp‐1ras, recommended glp‐1ras to switch to and the expected benefits of switching. Dose reductions & other considerations. Examples of drugs in this class include exenatide, liraglutide, dulaglutide, and semaglutide. Some also have documented cardiovascular benefit. This guide does not replace clinical judgment. The prescribing information for semaglutide states that patients should be initiated at 0.25 mg per week for 4 weeks,. Some are also approved for weight loss. In addition, an oral preparation of semaglutide (rybelsus®) has been licensed for use. Due to gl adverse events 3.1% 3.1% 6% (vs. Web published june 25, 2021. Researchers say this experimental drug increased. Ozempic (semaglutide) and victoza (liraglutide) are examples that treat type 2 diabetes. Summary of clinical evidence and comparison chart adverse effects: The researchers followed up the children of more than 50. N engl j med 2021;385:
Screenshot 20181019 12.58.25 My Diabetes Village

Comparison of GLP1receptor agonists Download Table

Evolution of GLP‐1 Receptor Agonists for Diabetes Treatment Biopharma PEG
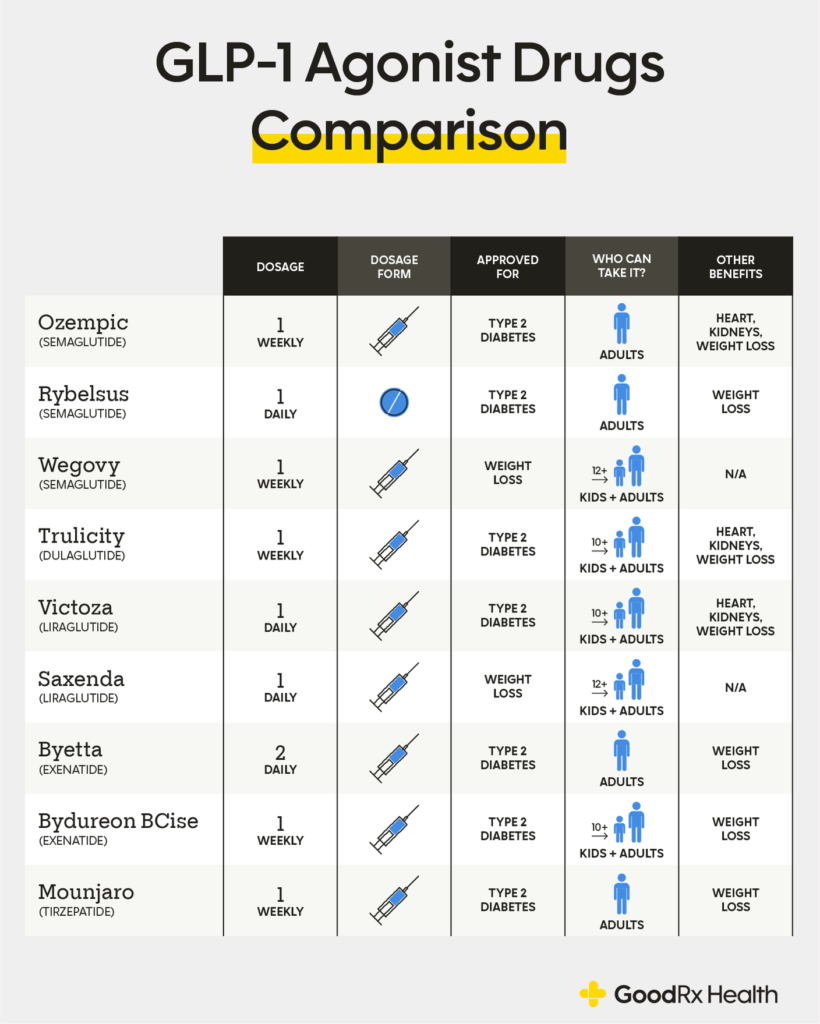
Glp 1 Receptor Agonist Comparison Chart
GLP 1 Agonist Conversion Chart

GLP 1 Agonist Conversion Chart

Glp1 Conversion Chart

Comparing GLP1 Agonists for Weight Loss Dr. Brian Yeung, ND
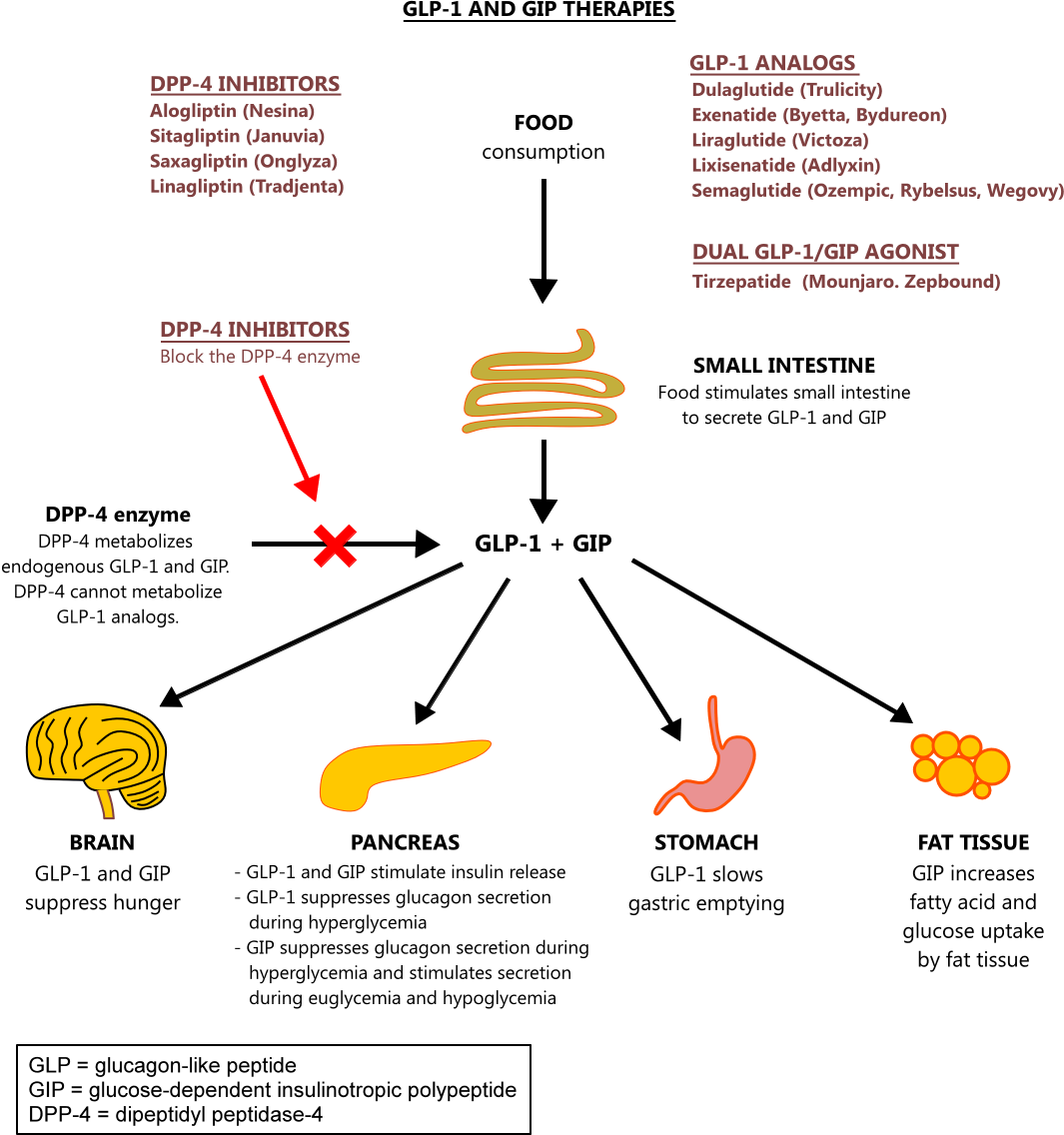
GLP1 analog dosing chart
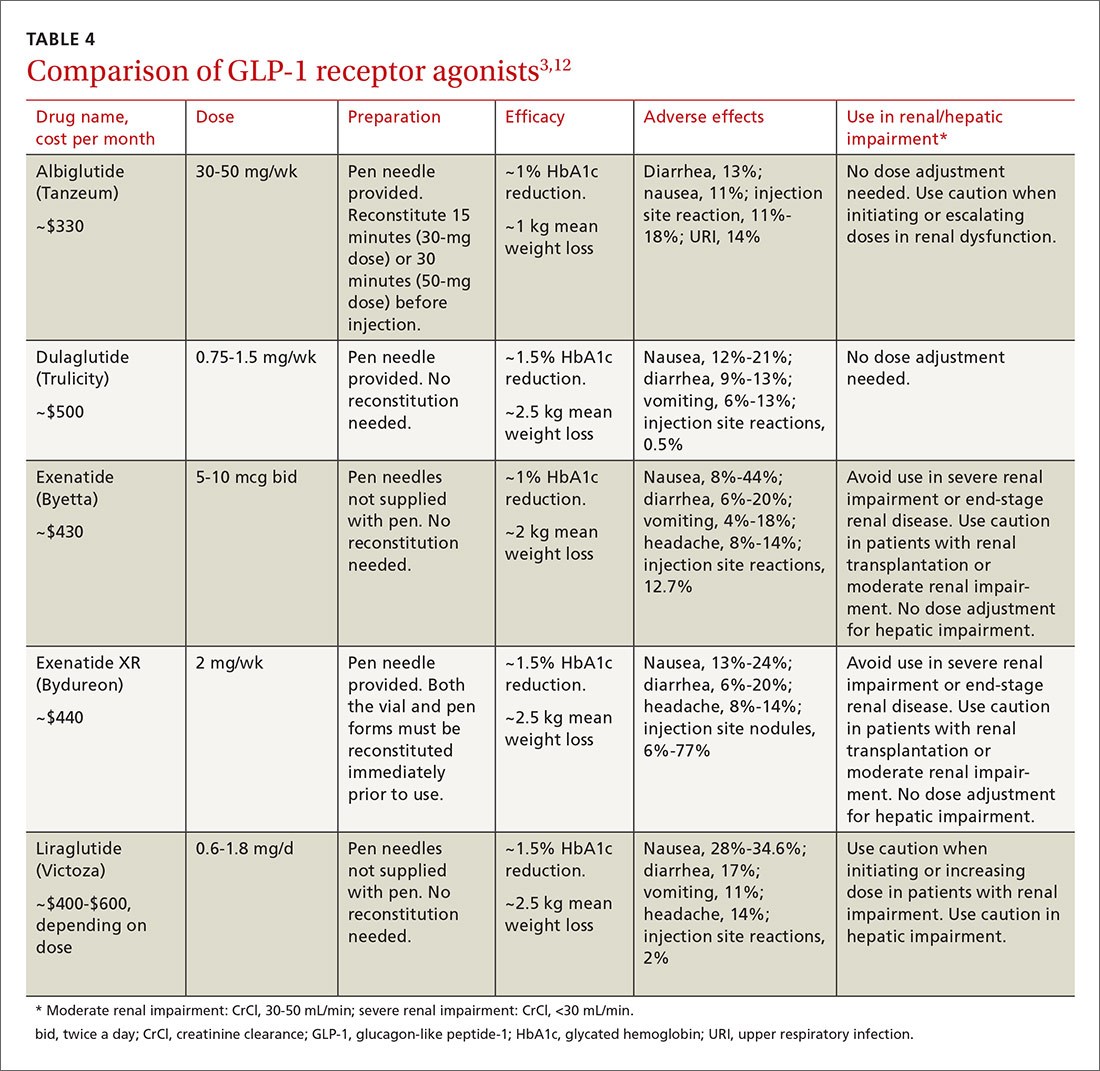
What next when metformin isn't enough for type 2 diabetes? MDedge
Dulaglutide (Trulicity®) Exenatide (Byetta®) Exenatide (Bydureon Bcise®) ᴾ.
A Weight Reduction Of ≥3% (Of Initial Body Weight) Additional Information About Dosing And Licensed Combinations, Precautions And Special Warning For Use Can Be Found At Www.medicines.org.uk.
Drugs Are Listed Alphabetically When Specific Drugs Are Suggested, The Order In The Table Is Therefore Not An Order Of Preference.
An Oral Option Is Also Available.
Related Post: