Ester Smells Chart
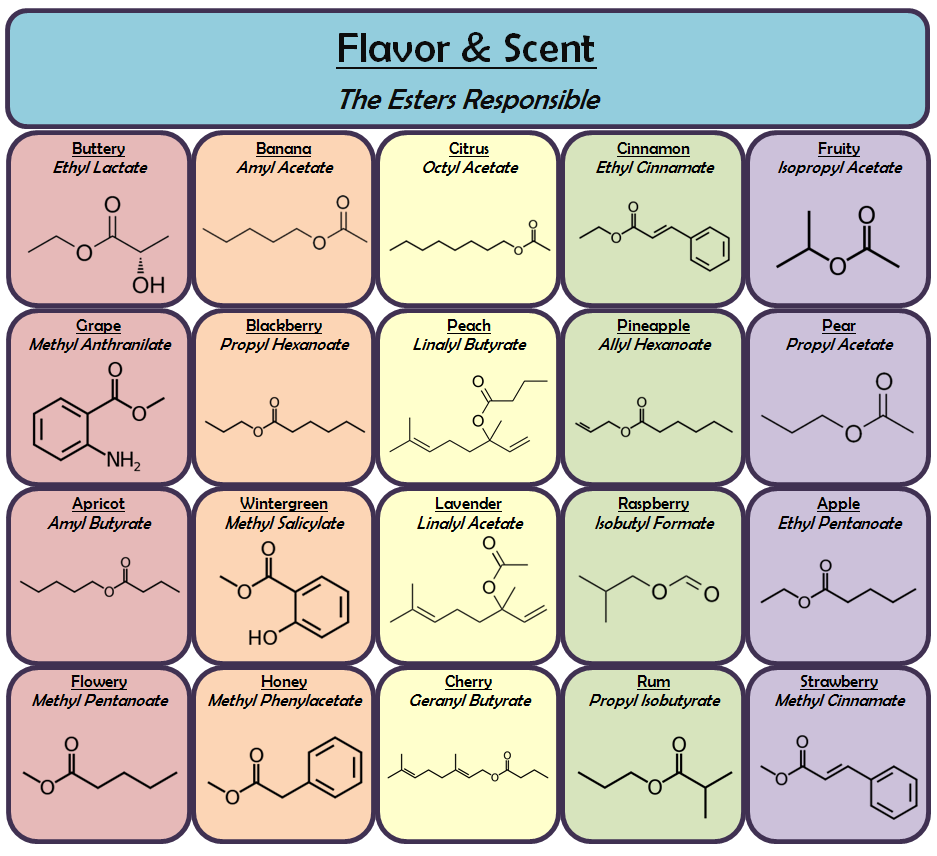
Ester Smells Chart - Web goal is to identify the ester that produces the wintergreen, banana, and cherry smells. Both natural and synthetic esters are used in perfumes and as flavouring agents. Web esters with low molar masses are generally sweet smelling, but as their molar mass is increased, they become odorless. Web list of ester odorants. Esters occur widely in nature. Asked 9 years, 5 months ago. Web this page defines esters and discusses their simple physical properties such as solubility and boiling points. Table of biologically compounds press their smells (250+ smells!) my foremost physics alphabet. Web table of esters or their smells; With reference to the first ones, different combinations of alcohols and carboxylic acids give rise to different esters, and each ester has a unique aroma. Predict the ester product to be made in each reaction. Hold your nose and read. But why do they actually smell? Nail polish remover, model paint, model airplane glue, pears: Web the esters shown here are ethyl acetate (a) and methyl butyrate (b). Table of esters and their smells. Heptyl 7 carbons octyl 8 carbons nonyl 9 carbons methanoate 1. Esters occur widely in nature. Be able to identify the ester, carboxylic acid, and alcohol functional groups. Web this page defines esters and discusses their simple physical properties such as solubility and boiling points. Bigger esters are important components of food flavouring and of perfumes. Once a flower or fruit has been chemically analyzed, flavor chemists can attempt to duplicate the natural odor or taste. They are responsible for the smell and taste of many flowers and fruits and can also be found in pheromones. Nail polish remover, model paint, model airplane glue, pears:. Web the esters shown here are ethyl acetate (a) and methyl butyrate (b). What is the reason behind its aromatic character? From the alcohol (first word) methanoate. Web smells and uses of esters. Ethyl ethanoate is important as a solvent. Web after completing this experiment, the student should be able to: Table of biologically compounds press their smells (250+ smells!) my foremost physics alphabet. With reference to the first ones, different combinations of alcohols and carboxylic acids give rise to different esters, and each ester has a unique aroma. Esters occur widely in nature. Salicylic acid (solid) and methanol (liquid). $75 buy now (blue set) $75 buy right (pink set) meet the terpenes: Web this page defines esters and discusses their simple physical properties such as solubility and boiling points. Esters occur widely in nature. Web they smell really nice, even though the two components that combine to form esters can smell like foot odor or vomit. Web table of. Web unlike carboxylic acids, esters generally have pleasant odours and are often responsible for the characteristic fragrances of fruits and flowers. Table of biologically compounds press their smells (250+ smells!) my foremost physics alphabet. Web unlike carboxylic acids, esters generally have pleasant odors and are often responsible for the characteristic fragrances of fruits and flowers. Modified 7 years, 6 months. $75 buy now (blue set) $75 buy right (pink set) meet the terpenes: Web why do esters actually smell? Web unlike carboxylic acids, esters generally have pleasant odours and are often responsible for the characteristic fragrances of fruits and flowers. Esters of various sorts are responsible for the smell and taste of all sorts of fruit. Table of esters and. Nail polish remover, model paint, model airplane glue, pears: Be able to systematically name esters. $75 buy now (blue set) $75 buy right (pink set) meet the terpenes: A visual introduced from isoprene to latex; Web the esters shown here are ethyl acetate (a) and methyl butyrate (b). Once a flower or fruit has been chemically analyzed, flavour chemists can attempt to duplicate the natural odour or taste. Both natural and synthetic esters are used in perfumes and as flavouring agents. Use proper laboratory technique for identifying odors. Nail polish remover, model paint, model airplane glue, pears: Bigger esters are important components of food flavouring and of perfumes. Be able to identify the ester, carboxylic acid, and alcohol functional groups. What is the reason behind its aromatic character? Web table of esters and their smells. This has also led to their common use in artificial flavorings and fragrances which aim to mimic those odors. Table of esters and their smells. Web unlike carboxylic acids, esters generally have pleasant odors and are often responsible for the characteristic fragrances of fruits and flowers. They are responsible for the smell and taste of many flowers and fruits and can also be found in pheromones. Web goal is to identify the ester that produces the wintergreen, banana, and cherry smells. Both natural and synthetic esters are used in perfumes and as flavoring agents. Web the esters shown here are ethyl acetate (a) and methyl butyrate (b). Web esters with low molar masses are generally sweet smelling, but as their molar mass is increased, they become odorless. Esters of various sorts are responsible for the smell and taste of all sorts of fruit. Salicylic acid (solid) and methanol (liquid) ester b: Once a flower or fruit has been chemically analyzed, flavor chemists can attempt to duplicate the natural odor or taste. Once a flower or fruit has been chemically analyzed, flavour chemists can attempt to duplicate the natural odour or taste. Asked 9 years, 5 months ago.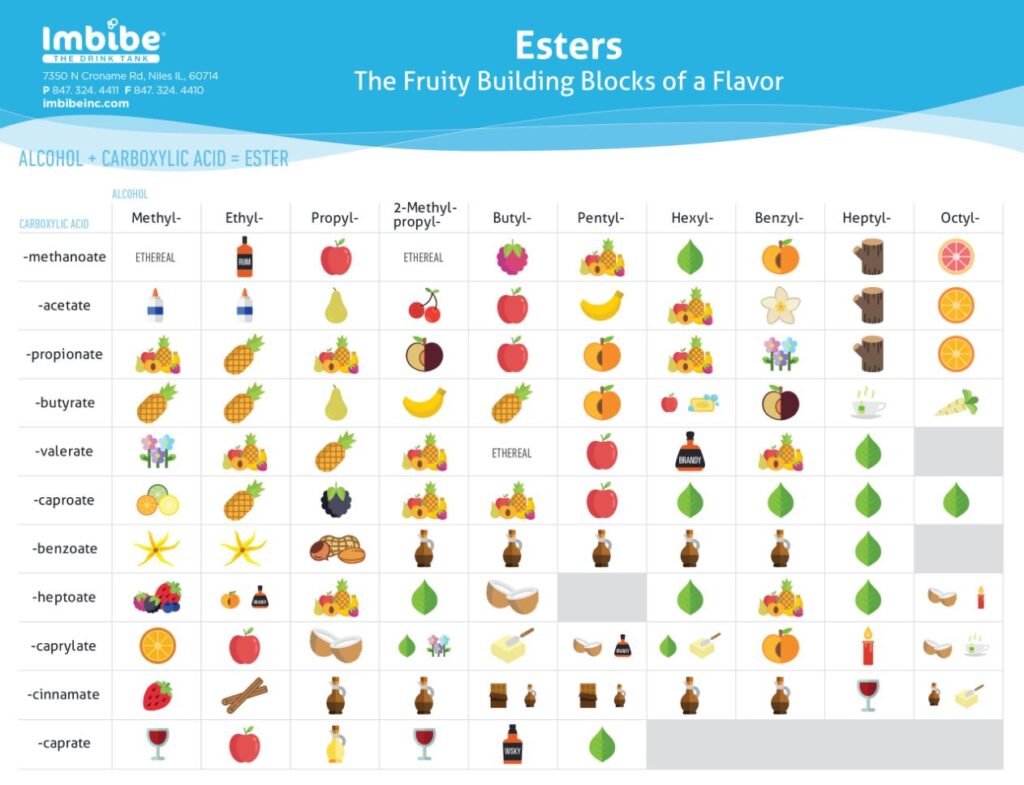
Esters The Fruity Building Blocks of Flavor Imbibe

Infographic Table of Esters and their Smells James Kennedy

ester smells chart
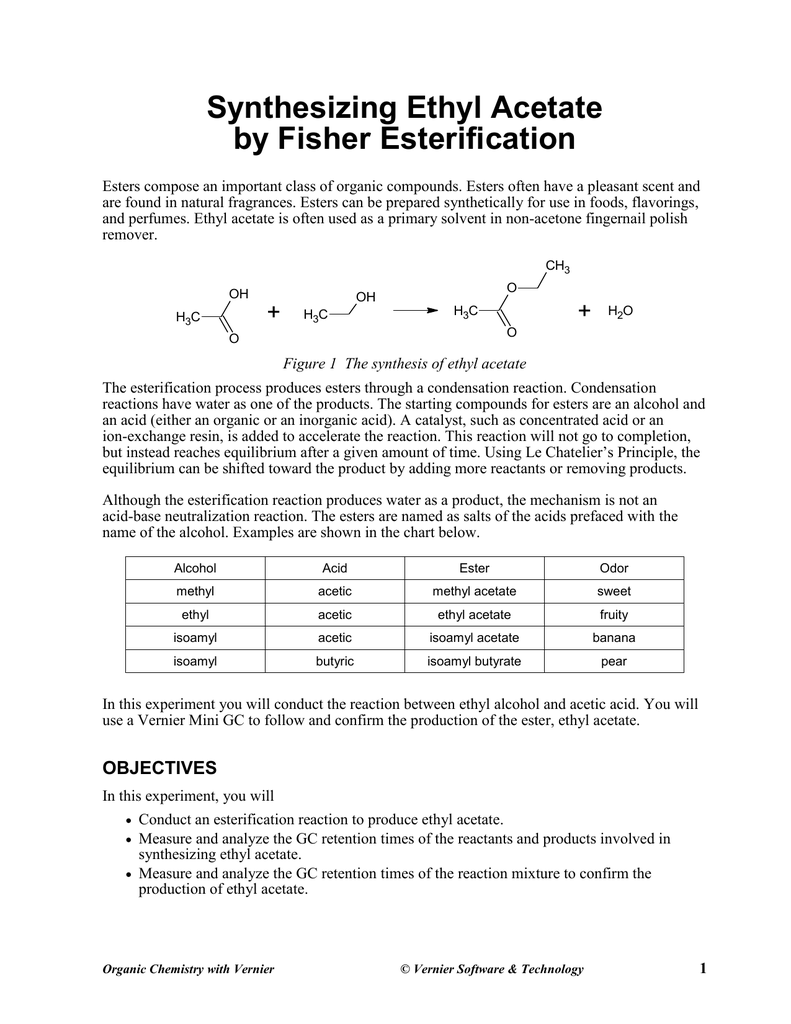
.jpg)
Ester Smell Chart Online Shopping
All Categories

ester

de geuren en smaken en hun chemische informatie Stooktips Stookforum
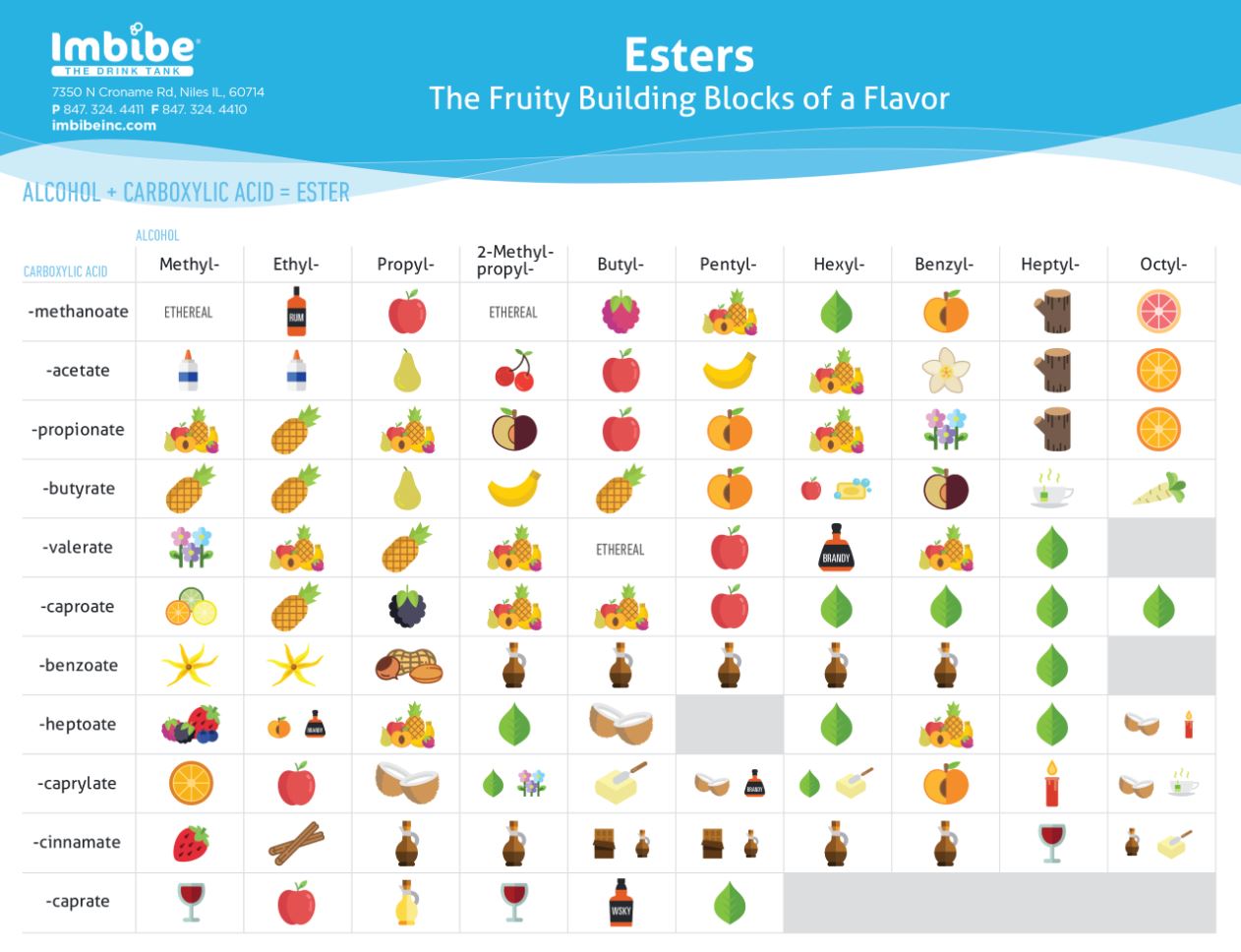
Esters The Fruity Building Blocks of Flavor Imbibe
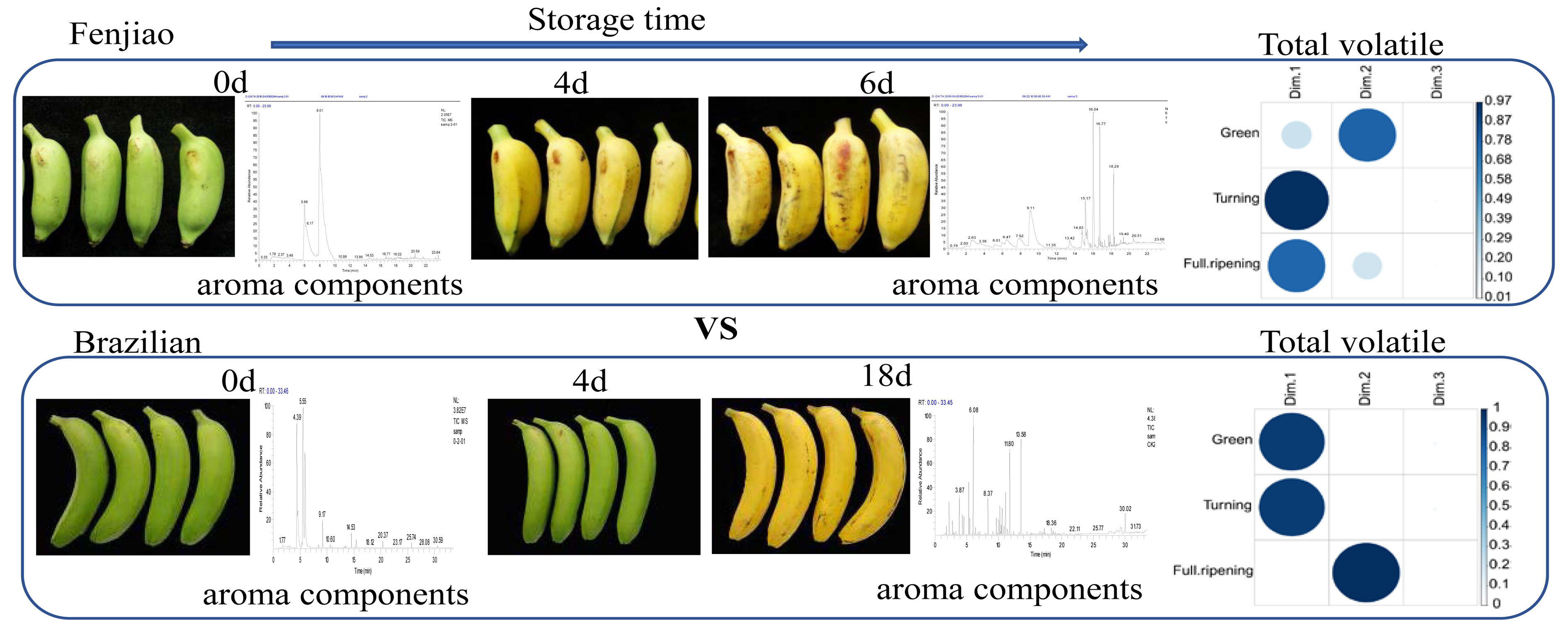
what ester smells like banana
Ester Smell Chart Online Shopping
Be Able To Systematically Name Esters.
Predict The Ester Product To Be Made In Each Reaction.
Both Natural And Synthetic Esters Are Used In Perfumes And As Flavouring Agents.
Modified 7 Years, 6 Months Ago.
Related Post: