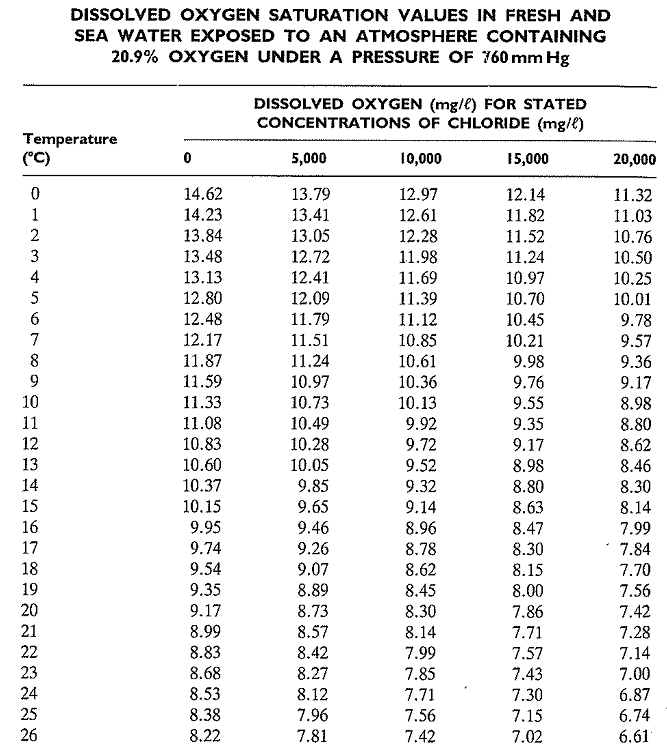
Dissolved Oxygen Saturation Chart
Dissolved Oxygen Saturation Chart - This page introduces the dissolved oxygen module, when to list dissolved oxygen as a candidate cause, ways to measure dissolved oxygen, simple and detailed conceptual model diagrams for. Dissolved oxygen (do) enters water by: Molecular view of dissolved oxygen occupying spaces between water molecules. P = barometric pressure, mm hg. As the chart shows, the concentration of dissolved oxygen in surface water is affected by temperature and has both a seasonal and a daily cycle. Healthy waters that can support life must contain dissolved oxygen (do). Web download scientific diagram | 7: The table was formulated in a laboratory using pure water. Why do we measure dissolved oxygen? Web to learn more about supersaturation, check out our technical note on environmental dissolved oxygen: Web last updated on february 29, 2024. Rapid movement from winds, waves, currents or mechanical aeration. It is an important measure of water quality as it indicates a water body's ability to support aquatic life. A few words about this chart: Web last updated on september 8, 2023. As the chart shows, the concentration of dissolved oxygen in surface water is affected by temperature and has both a seasonal and a daily cycle. The following table is based on zero salinity (for lakes), and 760 millimeters. Fresh water at 25oc = 8.26 mg/l. The ysi dissolved oxygen handbook is a practical guide to do in the lab or. The table was formulated in a laboratory using pure water. The values given are only approximations for estimating the oxygen content of a particular body of surface. Arterial oxygen saturation (sao 2) is commonly measured using pulse oximetry. P = barometric pressure, mm hg. Dissolved oxygen (do) is the amount of oxygen in water that is available to aquatic organisms. Dissolved oxygen (do) is the amount of oxygen in water that is available to aquatic organisms. Cold water can hold more dissolved oxygen than warm water. The standard unit of oxygen saturation is percent (%). Why do we measure dissolved oxygen? Dissolved oxygen (do) is the amount of oxygen that is present in water. It is an important measure of water quality as it indicates a water body's ability to support aquatic life. It contains helpful tips and recommendations to ensure you take accurate and repeatable do measurements. Do is necessary to support fish spawning, growth, and activity. If you are looking for a column of barometric pressure above 30.0 inches (760 mm) you. The values given are only approximations for estimating the oxygen content of a particular body of surface. Water bodies receive oxygen from. As the chart shows, the concentration of dissolved oxygen in surface water is affected by temperature and has both a seasonal and a daily cycle. Do is necessary to support fish spawning, growth, and activity. Web last updated. Fresh water at 25oc = 8.26 mg/l. Web however, it is easier to use an oxygen solubility chart. Dissolved oxygen concentrations may change dramatically with lake depth. However, the signal output from either sensor type will be identical in the two samples. Oxygen production occurs in the top portion of a lake, where sunlight drives the engines of photosynthesis. P = barometric pressure, mm hg. Healthy waters that can support life must contain dissolved oxygen (do). Do is one of the most commonly measured water quality parameters, but the reason for measuring it varies based on the environment. Water bodies receive oxygen from. Fresh water at 25oc = 8.26 mg/l. The values given are only approximations for estimating the oxygen content of a particular body of surface. Rapid movement from winds, waves, currents or mechanical aeration. Dissolved oxygen concentrations may change dramatically with lake depth. Water bodies receive oxygen from. 760 mm (29.92 inches) is standard pressure at sea level, and most sites in wisconsin are typically at elevations. Values greater than 100% air saturation. Web if two samples, one of fresh water and one of sea water, are fully saturated with oxygen the dissolved oxygen concentration will be: Cs = saturation solubility at given temperature from appendix, mg/l. Dissolved oxygen concentrations may change dramatically with lake depth. Do is one of the most commonly measured water quality parameters,. Arterial oxygen saturation (sao 2) is commonly measured using pulse oximetry. 0 5.0 ppt 9.0 ppt 10.0 ppt 18.1 ppt 15.0 ppt 27.1 ppt 20.0 ppt 36.1 ppt 25.0 ppt 45.2 ppt 0.0 14.62 13.73 12.89 12.10. Why do we measure dissolved oxygen? Oxygen saturation can be measured regionally and noninvasively. Percent saturation refers to the amount of do (percentage) dissolved in the water relative to the total amount possible. This value can then be multiplied by the measured percent air saturation to calculate the dissolved oxygen concentration 7. This page introduces the dissolved oxygen module, when to list dissolved oxygen as a candidate cause, ways to measure dissolved oxygen, simple and detailed conceptual model diagrams for. It contains helpful tips and recommendations to ensure you take accurate and repeatable do measurements. Web maximum dissolved oxygen concentration saturation table. Values greater than 100% air saturation. Rapid movement from winds, waves, currents or mechanical aeration. The table was formulated in a laboratory using pure water. Find the percentage of dissolved oxygen in water at a given temperature and dissolved oxygen level. Dissolved oxygen (do) is the amount of oxygen that is present in water. C's = solubility at barometric pressure p and given temperature, mg/l. These charts show the dissolved oxygen concentration at 100% air saturation at varying temperatures, and salinities.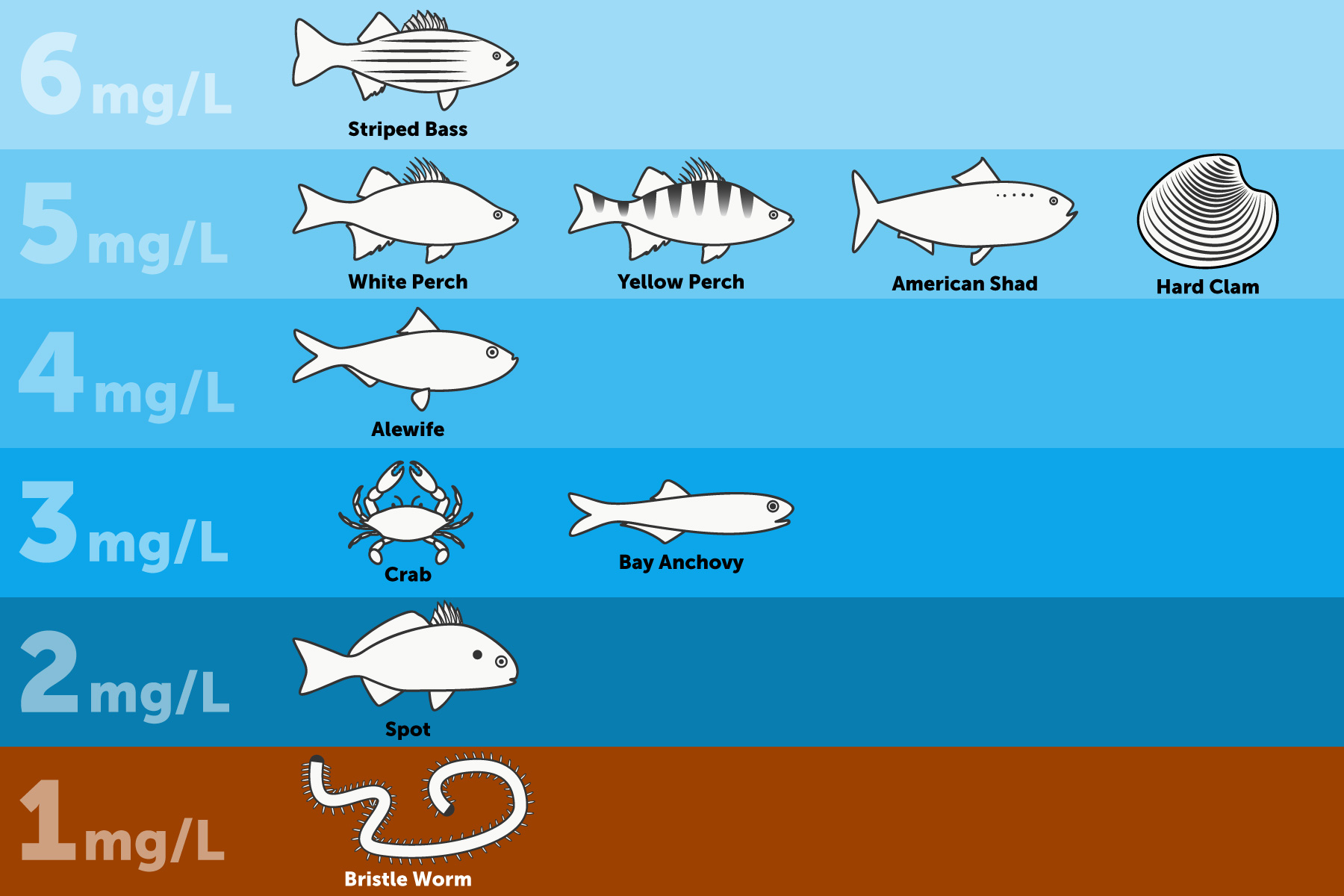
Dissolved Oxygen Chesapeake Bay Program

Dissolved Oxygen saturation () distribution with the associated MLD
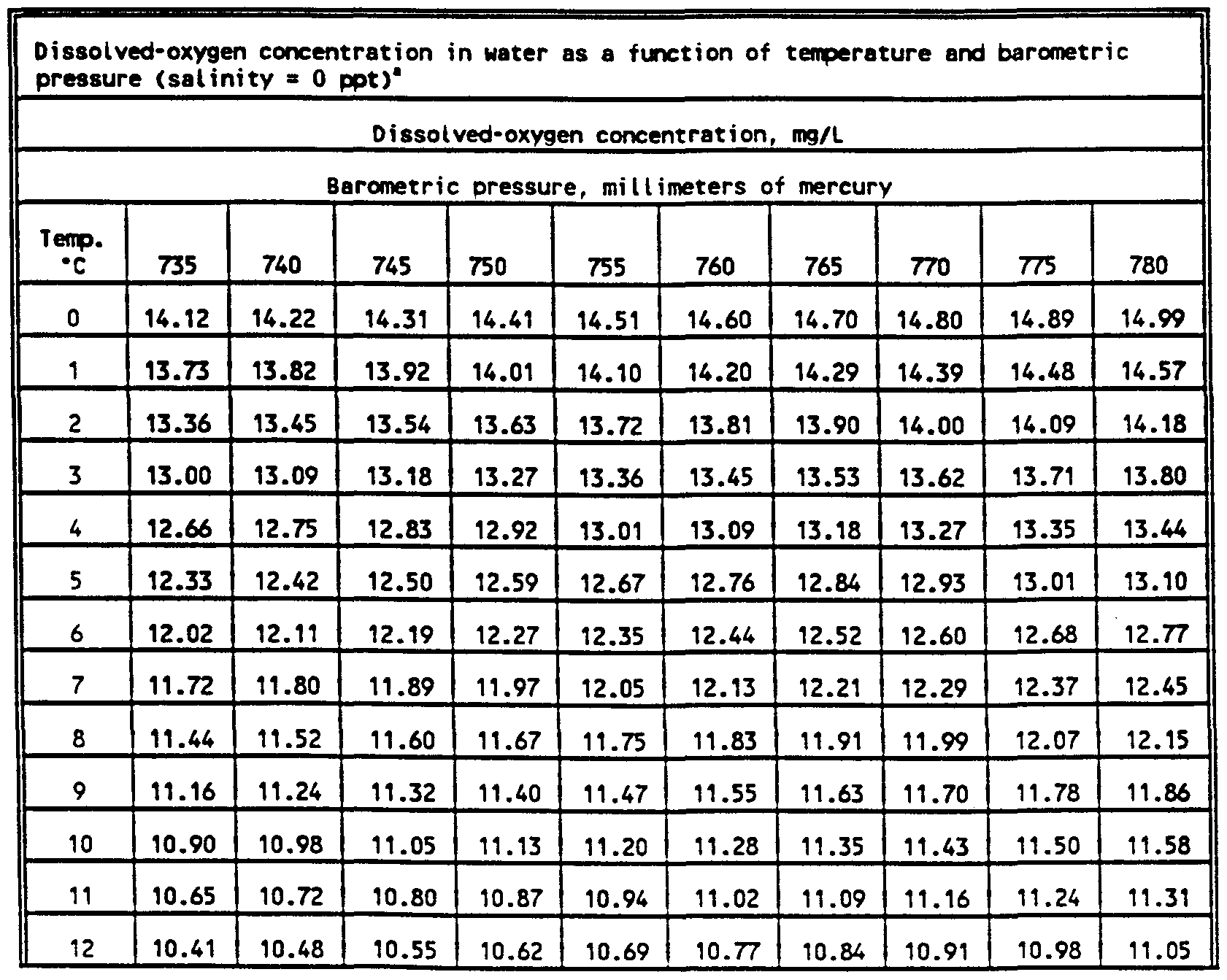
dissolved oxygen in water temperature table
DISSOLVED OXYGEN SATURATION VALUES IN FRESH AND SEA

7 Oxygen Saturation Chart for Calculating Dissolved Oxygen in Water

1 Saturated dissolved oxygen concentrations vary with temperature
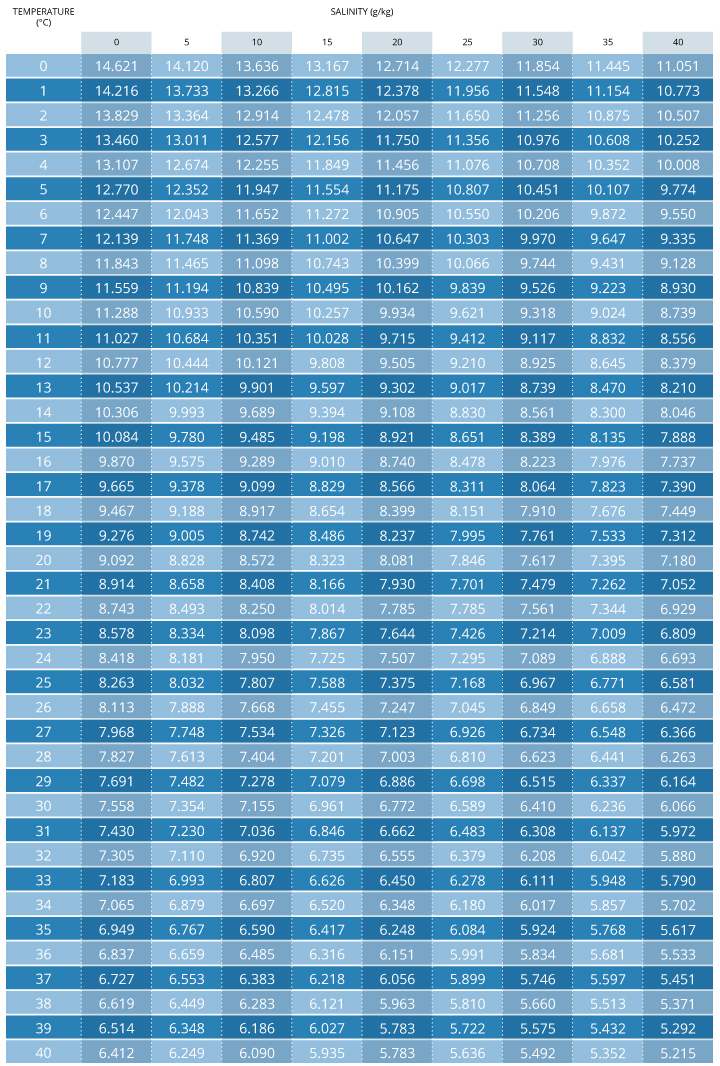
Dissolved Oxygen Environmental Measurement Systems

How Does Temperature Affect Dissolved Oxygen? Atlas Scientific

7 Oxygen Saturation Chart for Calculating Dissolved Oxygen in Water

Physicochemical data (water temperature, dissolved oxygen, air
(Do) In Water Is Influenced By A Number Of Factors, Including Water Temperature, Salinity And Atmospheric Pressure.
Molecular View Of Dissolved Oxygen Occupying Spaces Between Water Molecules.
However, The Signal Output From Either Sensor Type Will Be Identical In The Two Samples.
Web Last Updated On September 8, 2023.
Related Post: