Covalent Radii Chart
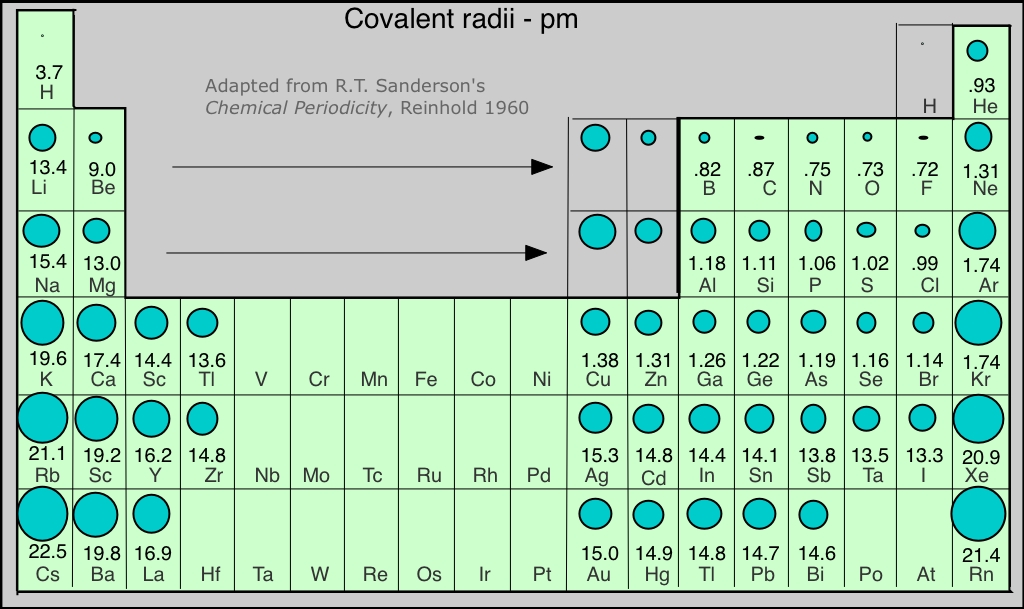
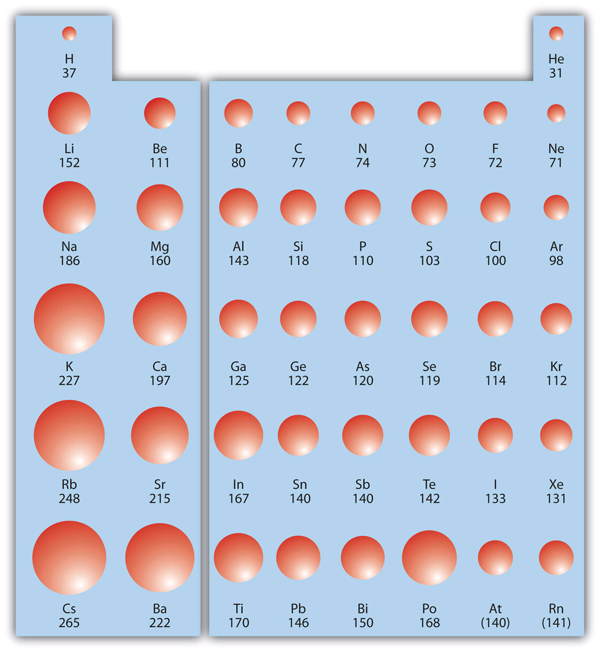
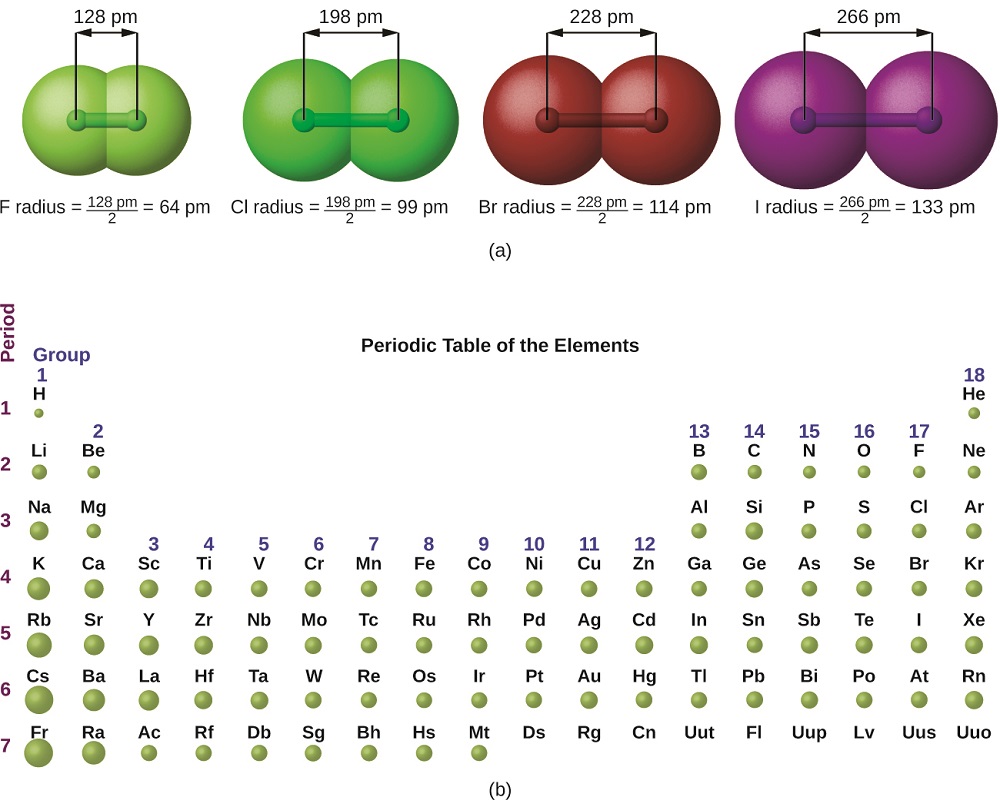
Covalent Radii Chart - Ionic radii are also available. The covalent radius, rcov, is a measure of the size of an atom that forms part of one covalent bond. The differences between the two single. 53 [citation needed] 120 or 110: Usually, you see covalent radius in units of picometers (pm) or. Radii are measured from bonds to c, n or o. Atomic radius of all the elements are mentioned in the chart below. These relationships are certainly not. A new set of covalent atomic radii has been deduced from crystallographic data for most of the elements with atomic numbers up to 96. The proposed radii show a well. Web these are he, ne, pm, at, rn, fr, ac, pa. Web atomic radius of all the elements (complete chart) march 23, 2023. Atomic radius of all the elements are mentioned in the chart below. Web the values of table 3 show the covalent radii from the two recent original determinations by cordero et al.[1], and pyykkö and atsumi [2].. Web this figure shows radii (in angstroms) of atoms and ions of the first four periods of the periodic table. Usually, you see covalent radius in units of picometers (pm) or. The differences between the two single. These relationships are certainly not. Web 119 rows covalent (single bond) covalent (triple bond) metallic 1: Covalent radii are in parentheses. Web in theory, the sum of two covalent radii should equal the covalent bond length between two atoms, but in practice the length of the bond depends on the. 53 [citation needed] 120 or 110: The covalent radius, rcov, is a measure of the size of an atom that forms part of one covalent bond.. It is usually measured either in picometres (pm) or angstroms (å), with 1 å = 100 pm. Web select from the following links to see visual periodicity representations for atomic radii, covalent radii, and van der waals radii. The differences between the two single. Web the covalent atomic radius (r cov) is half the internuclear distance in a molecule with. The proposed radii show a well. If the two atoms are of the same. Web this figure shows radii (in angstroms) of atoms and ions of the first four periods of the periodic table. Web the covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic. Web in theory, the sum of two covalent radii should equal the covalent bond length between two atoms, but in practice the length of the bond depends on the. Half of the single bond length between two similar atoms covalently bonded in a molecule is called the covalent radius. Ionic radii are also available. Usually, you see covalent radius in. These relationships are certainly not. The differences between the two single. Web the covalent radius is half the distance between two atoms that share a covalent bond. Elements above 96 are assigned a covalent radius and uncertainty of none. Web this is a set of covalent atomic radii (ref. The covalent radius, rcov, is a measure of the size of an atom that forms part of one covalent bond. Single bonds [1] single bonds [2] double bonds [2] triple bonds [2] 1: A new set of covalent atomic radii has been deduced from crystallographic data for most of the elements with atomic numbers up to 96. It is usually. You will see a selection of chart images that show different aspects of covalent radius. Elements above 96 are assigned a covalent radius and uncertainty of none. Usually, you see covalent radius in units of picometers (pm) or. Radii are measured from bonds to c, n or o. Web select from the following links to see visual periodicity representations for. Web 120 rows covalent radii; Web 119 rows covalent (single bond) covalent (triple bond) metallic 1: Usually, you see covalent radius in units of picometers (pm) or. In principle, the sum of the two covalent radii should equal the covalent bond length between two atoms, r(ab) = r(a) + r(b). These data show a well behaved periodic. These relationships are certainly not. Half of the single bond length between two similar atoms covalently bonded in a molecule is called the covalent radius. Web in this web page, you will find a covalent radius chart, a visual reference of charts. Moreover, different radii can be introduced for single, double and triple bonds (r1, r2 and r3 below), in a purely operational sense. The covalent radius, rcov, is a measure of the size of an atom that forms part of one covalent bond. Single bonds [1] single bonds [2] double bonds [2] triple bonds [2] 1: Elements above 96 are assigned a covalent radius and uncertainty of none. It is usually measured either in picometres (pm) or angstroms (å), with 1 å = 100 pm. Covalent radii are in parentheses. Radii from each element are listed from largest to smallest, ionic charge. Web 119 rows covalent (single bond) covalent (triple bond) metallic 1: Web in theory, the sum of two covalent radii should equal the covalent bond length between two atoms, but in practice the length of the bond depends on the. 53 [citation needed] 120 or 110: If the two atoms are of the same. You will see a selection of chart images that show different aspects of covalent radius. Web the values of table 3 show the covalent radii from the two recent original determinations by cordero et al.[1], and pyykkö and atsumi [2].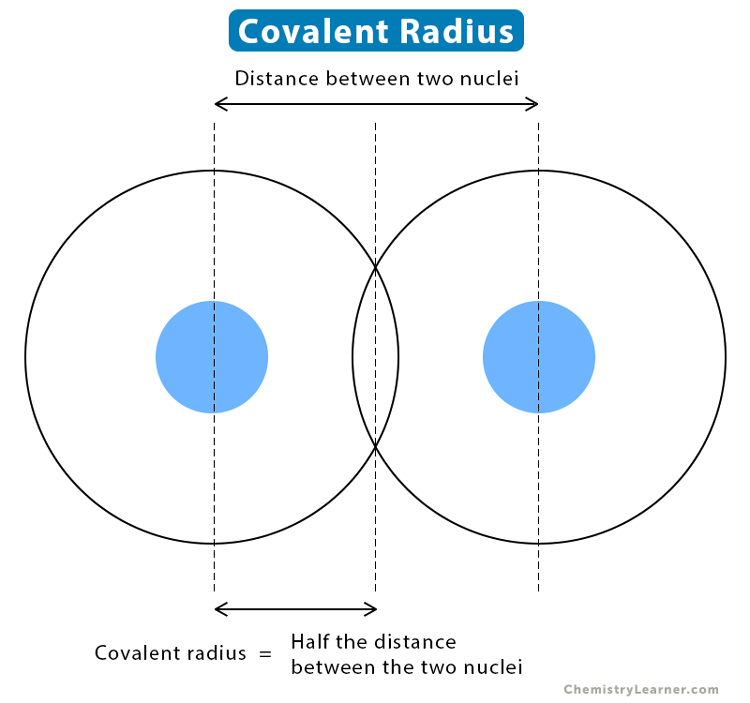
What is the Atomic Radius? EnthuZiastic
5.7 Periodic Properties of the Elements Chemistry LibreTexts
Periodic Trends Introductory Chemistry 1st Canadian Edition

Covalent Radius Definition and Trend
7.4 Electron Configurations, Valence Electrons, and the Periodic Table

Covalent Radius Definition and Trend
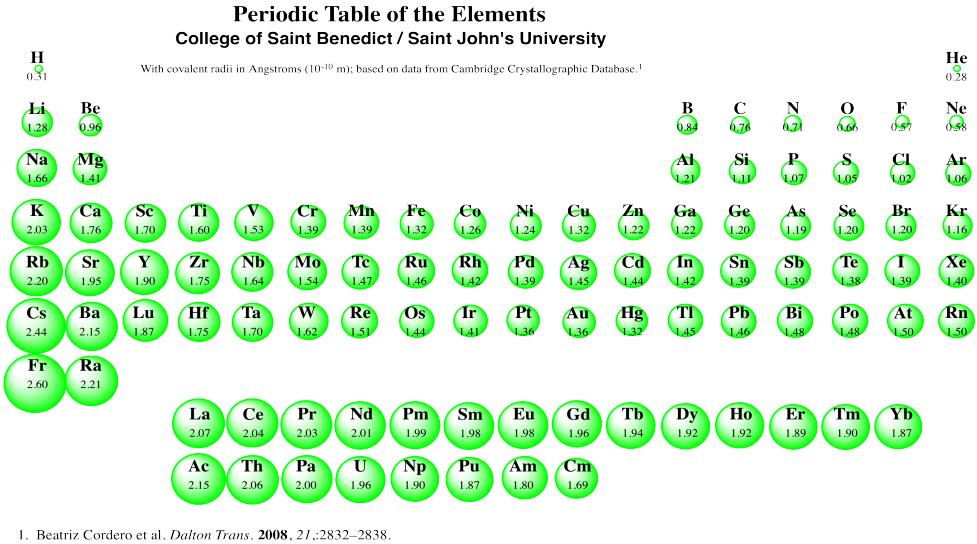
Structure & Reactivity Appendix Periodic Radii
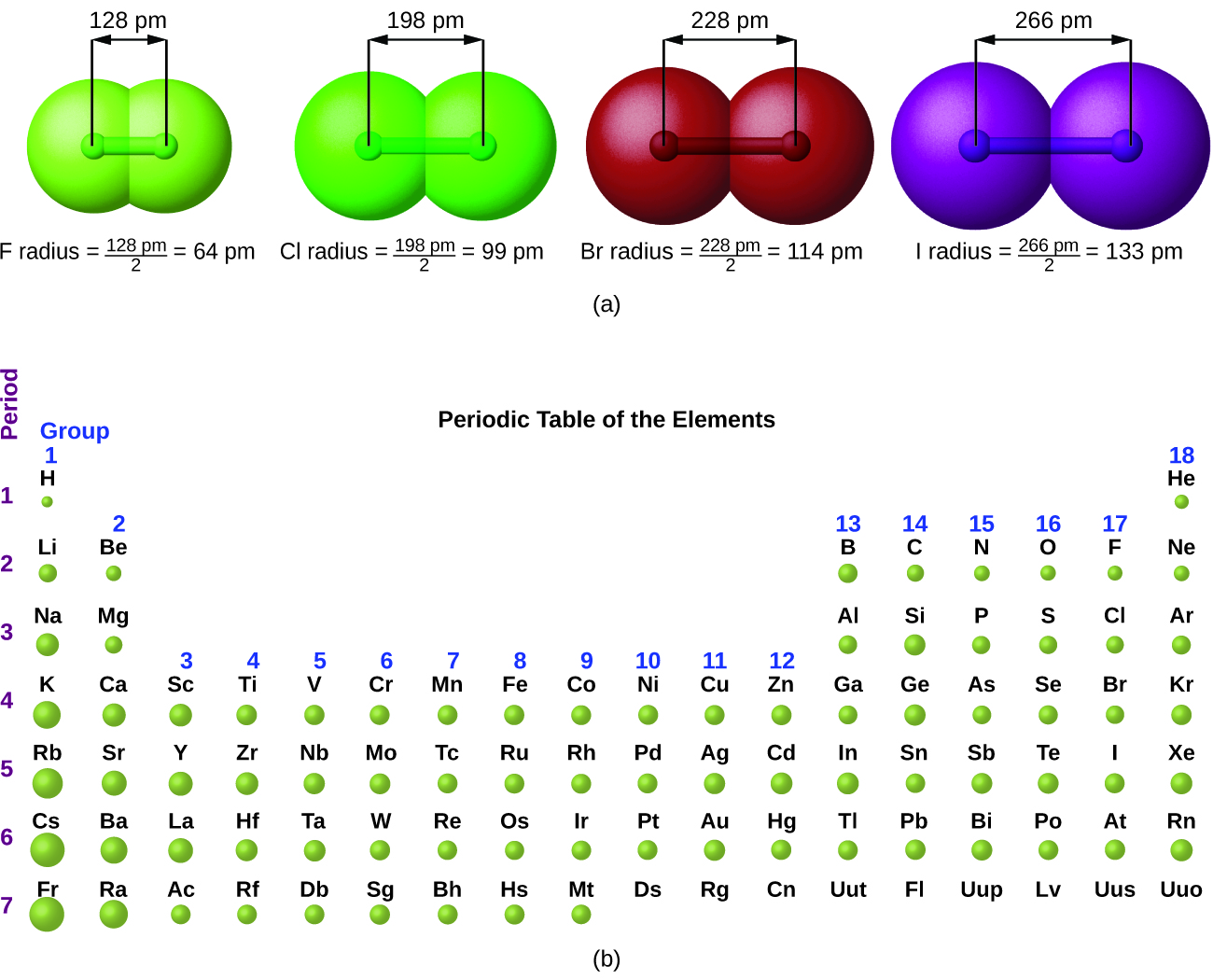
8.7 Periodic Trends and Variation of Properties General Chemistry

1.6 Periodic Variations in Element Properties Chemistry for

Elements, Atomic Radii and the Periodic Radii
Web Covalent Radius Increases As We Move Down A Group Because The N Level (Orbital Size) Increases.
Web The Covalent Atomic Radius (R Cov) Is Half The Internuclear Distance In A Molecule With Two Identical Atoms Bonded To Each Other, Whereas The Metallic Atomic Radius (R Met) Is.
Web This Figure Shows Radii (In Angstroms) Of Atoms And Ions Of The First Four Periods Of The Periodic Table.
These Data Show A Well Behaved Periodic.
Related Post: