Complete The Following Chart Of Gas Properties For Each Positive
Complete The Following Chart Of Gas Properties For Each Positive - Co2, h2, o2, hcl, nh3 Web postlab complete the following chart of gas properties. State the three major properties of gases that distinguish them from condensed phases of matter. Web 402 rows 9.1 gas pressure; Postlab complete the following chart of gas properties. Web make sure you thoroughly understand the following essential ideas: This answer supports our expectation from charles’s law, namely, that. For each positive observation, write a balanced chemical equation to. Web make sure you thoroughly understand the following essential ideas: Web make sure you thoroughly understand the following essential ideas: Using the ideal gas law \(\mathrm{pv=nkt (p=const)}\), fig 2 : The ideal gas equation, pv = nrt, is an equation used to predict the behavior of gases. Elements that exist as gases at room temperature and pressure are. Web as you have learned, ions are atoms or molecules bearing an electrical charge. 9.2 relating pressure, volume, amount, and temperature: It's used to calculate an unknown quantity when the other four are given. Web make sure you thoroughly understand the following essential ideas: Greg l via wikipedia ) this page titled 6:. Web for each positive observation, write a balanced chemical equation to describe the process. complete the following chart of gas properties. Co2, h2, o2, hcl, nh3 The reaction is nonspontaneous ( not spontaneous) at 25 °c. Web as you have learned, ions are atoms or molecules bearing an electrical charge. Postlab complete the following chart of gas properties. 9.2 relating pressure, volume, amount, and temperature: State the three major properties of gases that distinguish them from condensed phases of matter. Postlab complete the following chart of gas properties. Using the ideal gas law \(\mathrm{pv=nkt (p=const)}\), fig 2 : Complete the following chart of gas properties. If δv is positive, then w is positive, meaning that work is done by the gas on the outside world. Web as you have learned, ions are atoms or molecules bearing an electrical charge. The properties of gases (exercises) these are homework exercises to accompany chapter 16 of mcquarrie and simon's physical chemistry: Web as you have learned, ions are atoms or molecules bearing an electrical charge. State the three major properties of gases that distinguish them from condensed phases of matter. The ideal gas equation, pv = nrt, is an equation used to. Web rearranging and solving gives: Web we will start with covering basic gas properties and then learn several equations of state, which are mathematical equations that relate measurable values like the volume,. Web for each positive observation, write a balanced chemical equation to describe the process. complete the following chart of gas properties. If δv is positive, then w is. Web rearranging and solving gives: Web 402 rows 9.1 gas pressure; Web c2h6(g) h2(g) + c2h4(g) answer: State the three major properties of gases that distinguish them from condensed phases of matter. V2 = 0.300l×303 k 283 k = 0.321l v 2 = 0.300 l × 303 k 283 k = 0.321 l. Web make sure you thoroughly understand the following essential ideas: Web charles’s law states that the volume of a given amount of gas is directly proportional to its temperature on the kelvin scale when the pressure is held constant. Web learn how gas properties vary with volume, heat, and particle type in this interactive simulation. For each positive observation, write. Ced chemical gas appearance reaction. 9.3 stoichiometry of gaseous substances, mixtures, and reactions;. 9.2 relating pressure, volume, amount, and temperature: Web make sure you thoroughly understand the following essential ideas: The ideal gas equation, pv = nrt, is an equation used to predict the behavior of gases. Web gases have the lowest density of the three, are highly compressible, and fill their containers completely. If δv is positive, then w is positive, meaning that work is done by the gas on the outside world. Elements that exist as gases at room temperature and pressure are. Web c2h6(g) h2(g) + c2h4(g) answer: The reaction is nonspontaneous ( not. Web charles’s law states that the volume of a given amount of gas is directly proportional to its temperature on the kelvin scale when the pressure is held constant. Web make sure you thoroughly understand the following essential ideas: Web rearranging and solving gives: Web as you have learned, ions are atoms or molecules bearing an electrical charge. Greg l via wikipedia ) this page titled 6:. The properties of gases (exercises) these are homework exercises to accompany chapter 16 of mcquarrie and simon's physical chemistry: Elements that exist as gases at room temperature and pressure are. Web make sure you thoroughly understand the following essential ideas: The reaction is nonspontaneous ( not spontaneous) at 25 °c. V2 = 0.300l×303 k 283 k = 0.321l v 2 = 0.300 l × 303 k 283 k = 0.321 l. Web make sure you thoroughly understand the following essential ideas: 9.3 stoichiometry of gaseous substances, mixtures, and reactions;. Ced chemical gas appearance reaction. Web we will start with covering basic gas properties and then learn several equations of state, which are mathematical equations that relate measurable values like the volume,. The standard free energy change for a reaction may also be. Web learn how gas properties vary with volume, heat, and particle type in this interactive simulation.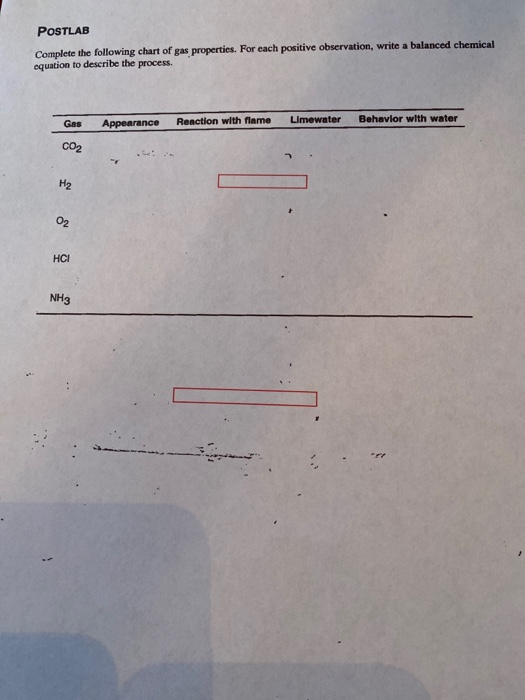
Solved POSTLAB Complete the following chart of gas
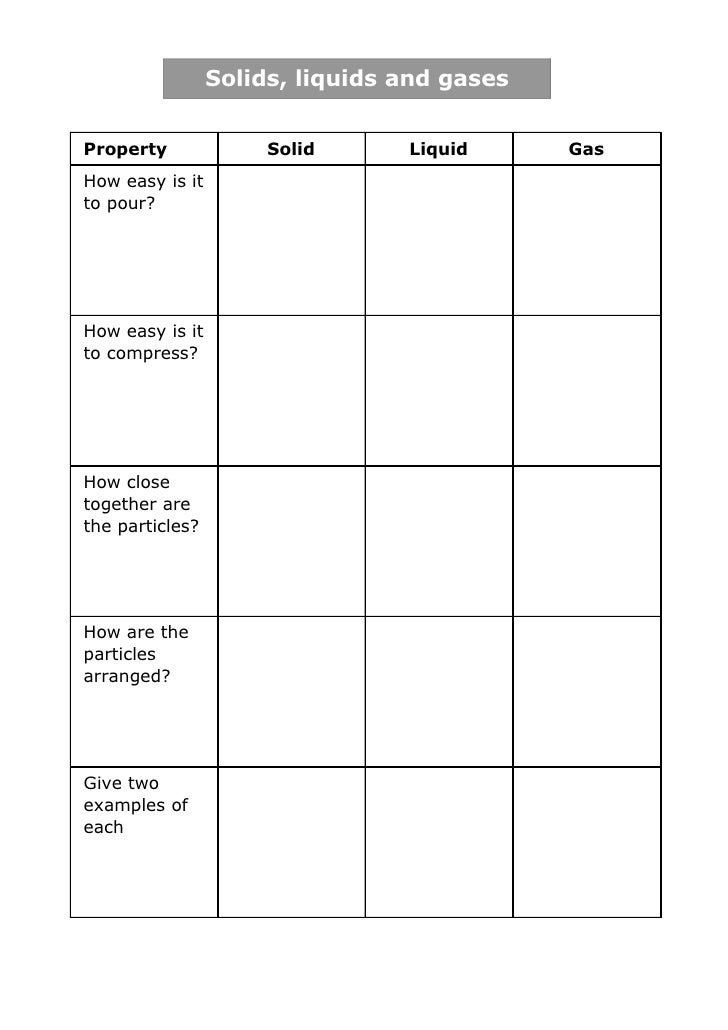
Solids, Liquids And Gases Fill The Table

Properties of gases Department Of Chemical Engineering

PPT Chapter 11 Gases PowerPoint Presentation, free download ID1586364
Solved POSTLAB Complete the following chart of gas

Common Gases and their properties O Level Secondary Chemistry Tuition
Solved Alt Ctrl POSTLAB Complete the following chart of gas

Gases Chart Scholars Labs
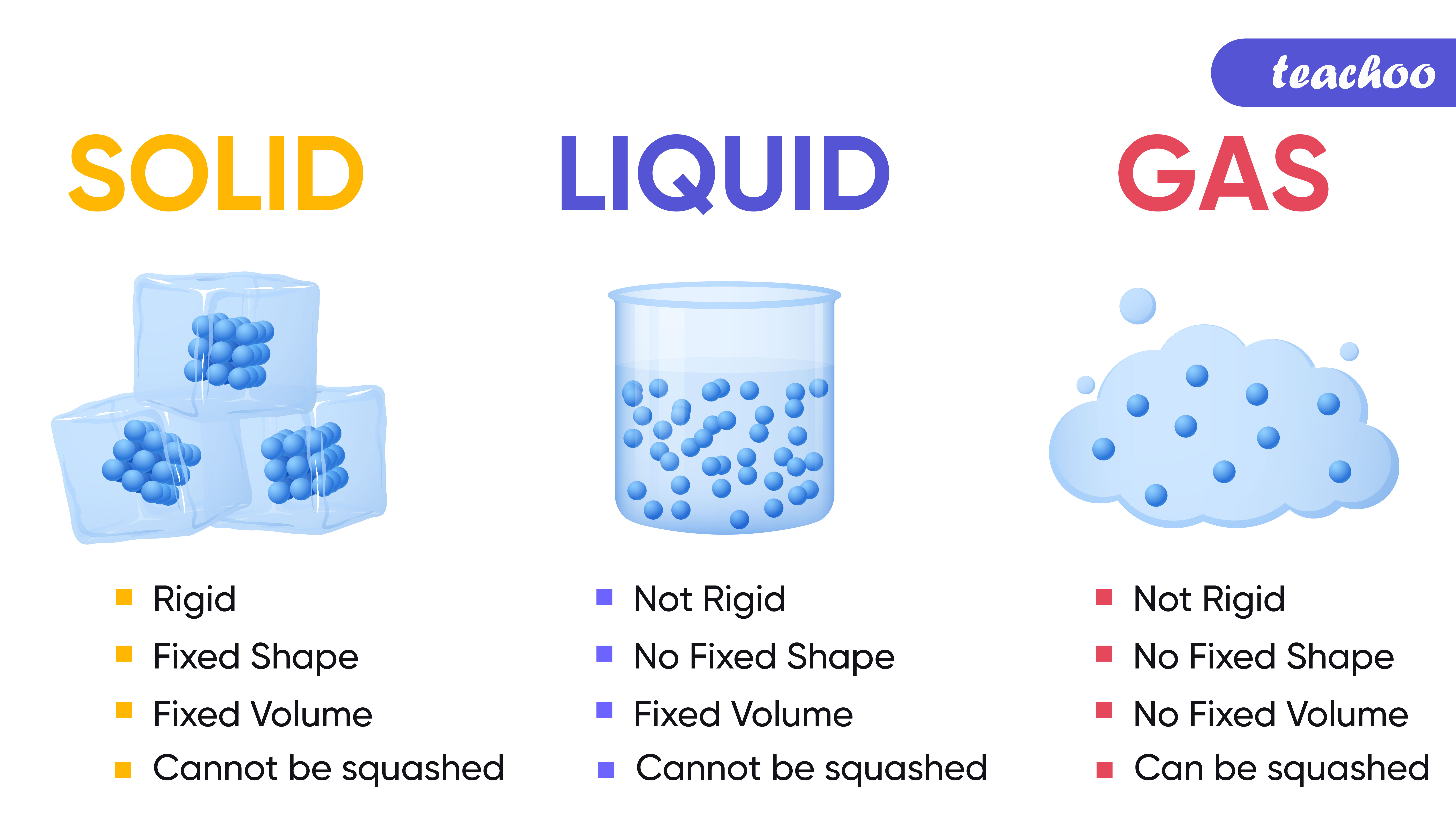
Solids Liquids Gases Chart
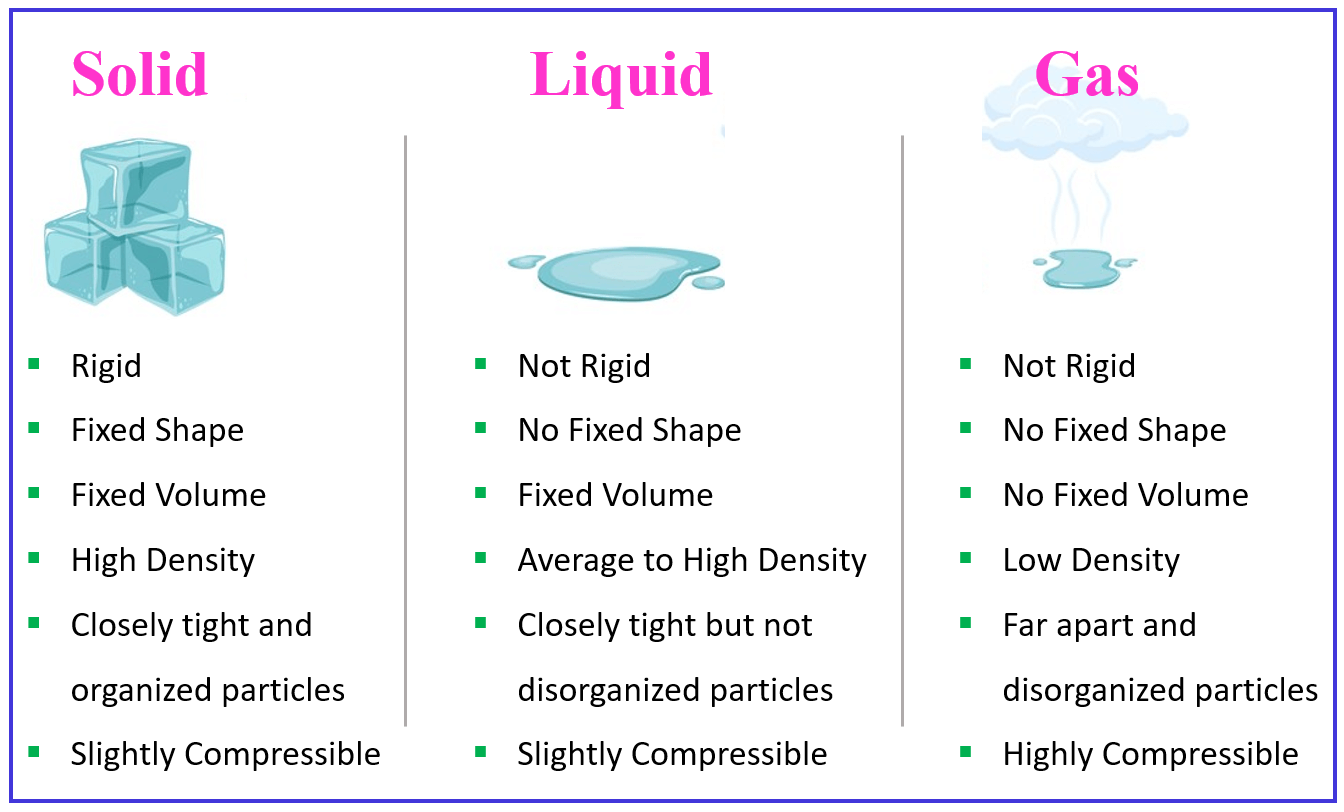
Solid Liquid Gas Plasma Chart
For Each Positive Observation, Write A Balan Equation To Describe The Process.
The Ideal Gas Equation, Pv = Nrt, Is An Equation Used To Predict The Behavior Of Gases.
Using The Ideal Gas Law \(\Mathrm{Pv=Nkt (P=Const)}\), Fig 2 :
Web 402 Rows 9.1 Gas Pressure;
Related Post:

