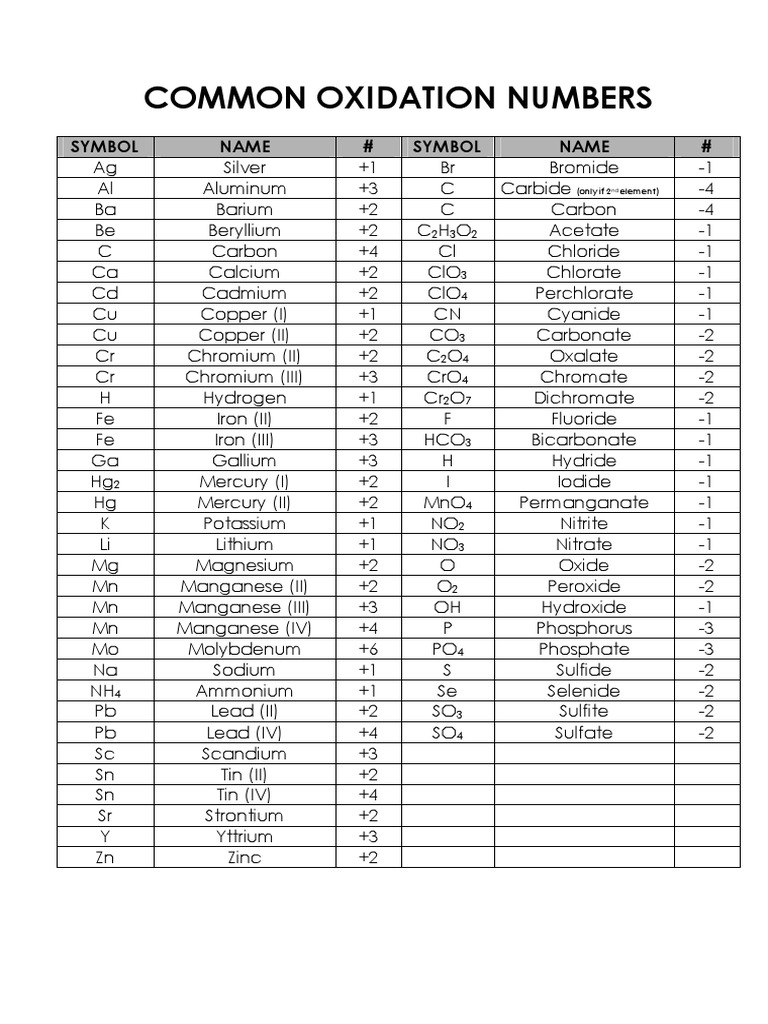
Chart Of Oxidation Number
Chart Of Oxidation Number - This periodic table contains the oxidation numbers of the elements as well as element numbers, symbols, names, and atomic weights. Remember, shells don’t neatly stack on top of each other, so valence (and oxidation state) may not be the same as the total number of electrons in the outer shell. Organic compounds and some covalent compounds do not have oxidation states assigned to the atoms in the. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. We can use oxidation numbers to keep track of where electrons are in a molecule, and how they move during a reaction. If you're working out the oxidation states of the atoms in a reaction and you get one that's not on this chart, it's probably worth checking your work. Oxidation number of an atom when an element has combined with the same element. Elements have an oxidation state of zero, and atoms in ionic compounds are usually assigned a positive or negative oxidation state. Web this printable periodic table contains the number, symbol, name, atomic mass and oxidation states of each element. Web what if you kept on adding electrons to the element? The most common oxidation states are in bold text and predicted or unconfirmed states are in italics. Web determine what is the oxidizing and reducing agents in the following reaction. Web enter the formula of a chemical compound to find the oxidation number of each element. Web in simple words, the oxidation number is the number assigned to the components. You can download the chart and the table above by clicking on either. This table is available for download as a pdf file and printed for offline use. Web an oxidation number is a number that is assigned to an atom to indicate its state of oxidation or reduction during a chemical reaction. The most common oxidation states are in. Oxidation number of an atom when an element has combined with the same element. Web enter the formula of a chemical compound to find the oxidation number of each element. Oxidation numbers of according to the atomic number, first 20 elements. In addition, the electron configurations of neutral atoms (oxidation state zero; Information of oxidation numbers of monoatomic ions in. Oxidation number of an atom when an element has combined with the same element. Web an oxidation number is a number that is assigned to an atom to indicate its state of oxidation or reduction during a chemical reaction. Web determine what is the oxidizing and reducing agents in the following reaction. Web the oxidation number is a positive or. As the table shows, the presence of the other oxidation states varies, but follows some patterns. Enter just an element symbol to show the common and uncommon oxidation states of the element. Information of oxidation numbers of monoatomic ions in periodic table. Zn + 2h+ zn2+ +h2 zn + 2 h + zn 2 + + h 2. The oxidation. Elements have an oxidation state of zero, and atoms in ionic compounds are usually assigned a positive or negative oxidation state. Lih, nah, and cah 2. Oxidation numbers of according to the atomic number, first 20 elements. This is a list of all the known oxidation states of the chemical elements, excluding nonintegral values. Oxidation states of s block. Elements have an oxidation state of zero, and atoms in ionic compounds are usually assigned a positive or negative oxidation state. Use uppercase for the first character in the. Oxidation numbers of according to the atomic number, first 20 elements. Web the oxidation number is the positive or negative number of an atom that indicates the electrical charge the atom. Web the following table lists the preferred (in bold), occasionally occurring, observed under certain conditions (in parentheses) and theoretically predicted oxidation states (in brackets) of the various chemical elements; The oxidation number of hydrogen is +1 when it is combined with a nonmetal. Web determine what is the oxidizing and reducing agents in the following reaction. A net ionic charge. You can't actually do that with vanadium, but you can with an element like sulphur. Enter just an element symbol to show the common and uncommon oxidation states of the element. This table is available for download as a pdf file and printed for offline use. Information of oxidation numbers of monoatomic ions in periodic table. As the table shows,. The oxidation number, in simple terms, can be described as the number that is allocated to elements in a chemical combination. First previous 1 next last. This table is available for download as a pdf file and printed for offline use. The most common oxidation states are in bold text and predicted or unconfirmed states are in italics. Lih, nah,. Web the oxidation number represents how many electrons an atom has gained or lost in a molecule. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. Web what is the oxidation number when an element has not combined or do not form a compound. Oxidation numbers of according to the atomic number, first 20 elements. To keep track of electrons in a. Web enter the formula of a chemical compound to find the oxidation number of each element. Organic compounds and some covalent compounds do not have oxidation states assigned to the atoms in the. The oxidation number is basically the count of electrons that atoms in a molecule can share, lose or gain while forming chemical bonds with other atoms of a different element. First previous 1 next last. Web the following table lists the preferred (in bold), occasionally occurring, observed under certain conditions (in parentheses) and theoretically predicted oxidation states (in brackets) of the various chemical elements; The most common oxidation states are in bold text and predicted or unconfirmed states are in italics. Web charges given to atoms in a molecule in this way are called oxidation numbers. Elements have an oxidation state of zero, and atoms in ionic compounds are usually assigned a positive or negative oxidation state. You can download the chart and the table above by clicking on either. This table is available for download as a pdf file and printed for offline use. In our water example, hydrogen is assigned an oxidation number of +1 because each individual hydrogen has lost one electron.
oxidation states Archives Science Notes and Projects
Common Oxidation Numbers Chart
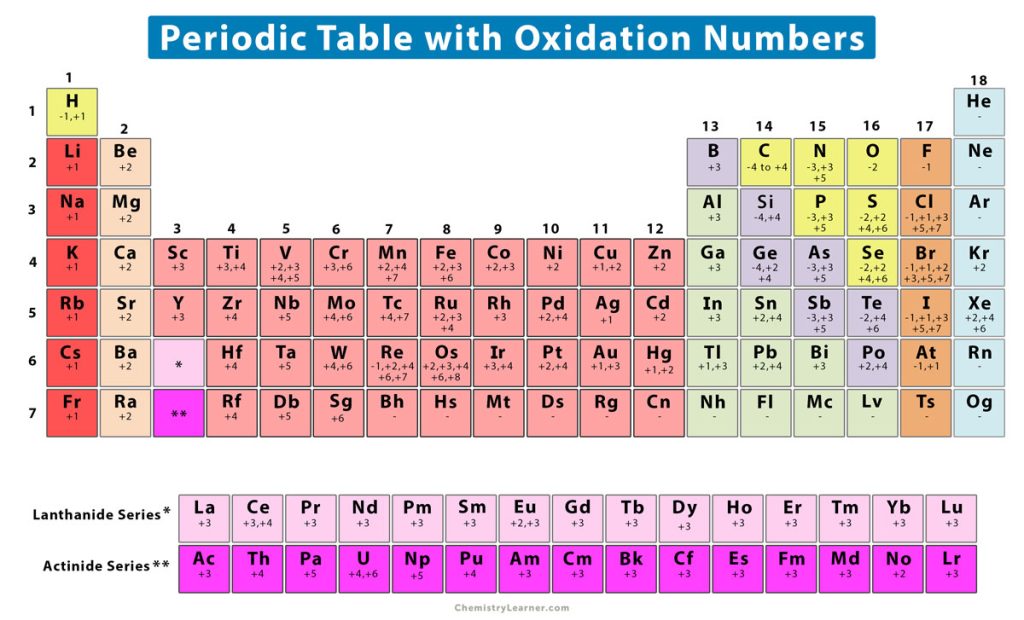
Oxidation Number (State) Definition, Rules, How to Find, and Examples
Oxidation Numbers Periodic Table Elements

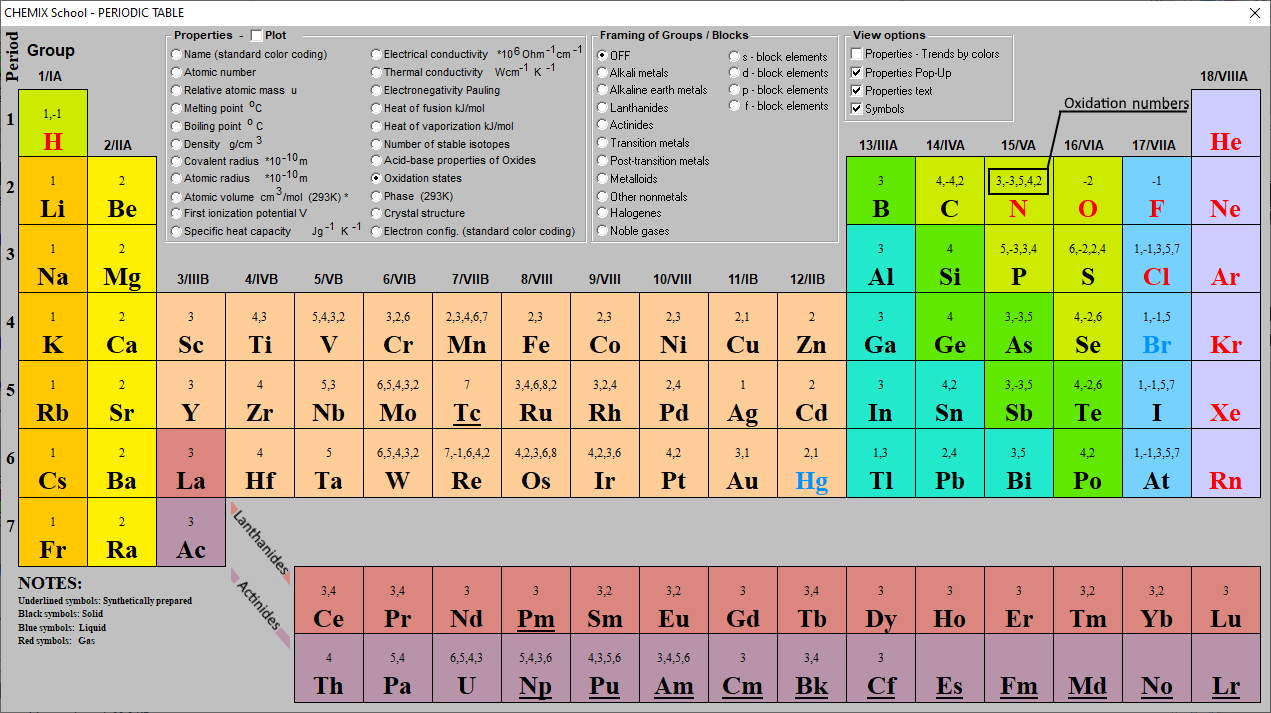
Downloadable Periodic Table Oxidation States
Periodic Table Oxidation Chart
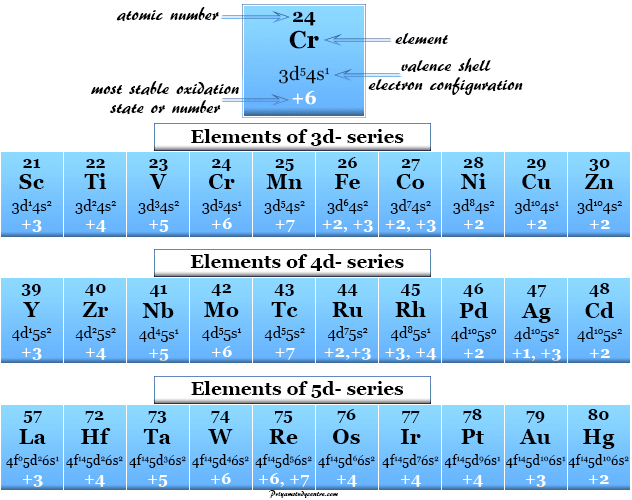
Oxidation Number Periodic table elements Definition, Rules (2023)

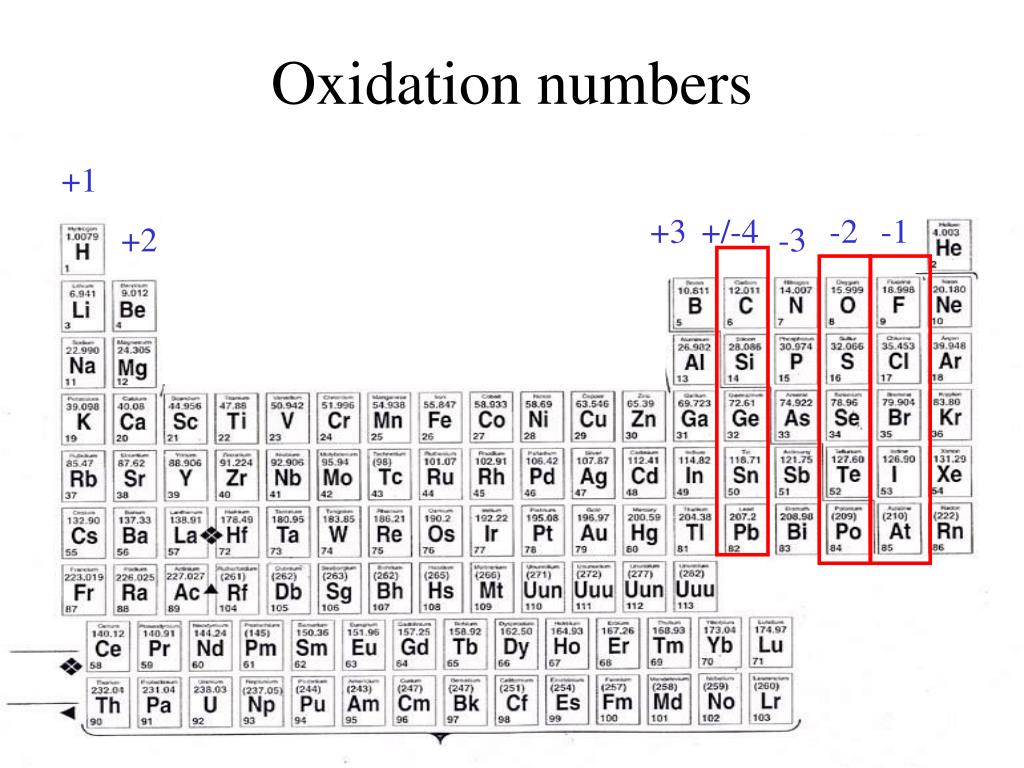
Labeled Periodic Table With Oxidation Numbers Periodic Table Timeline
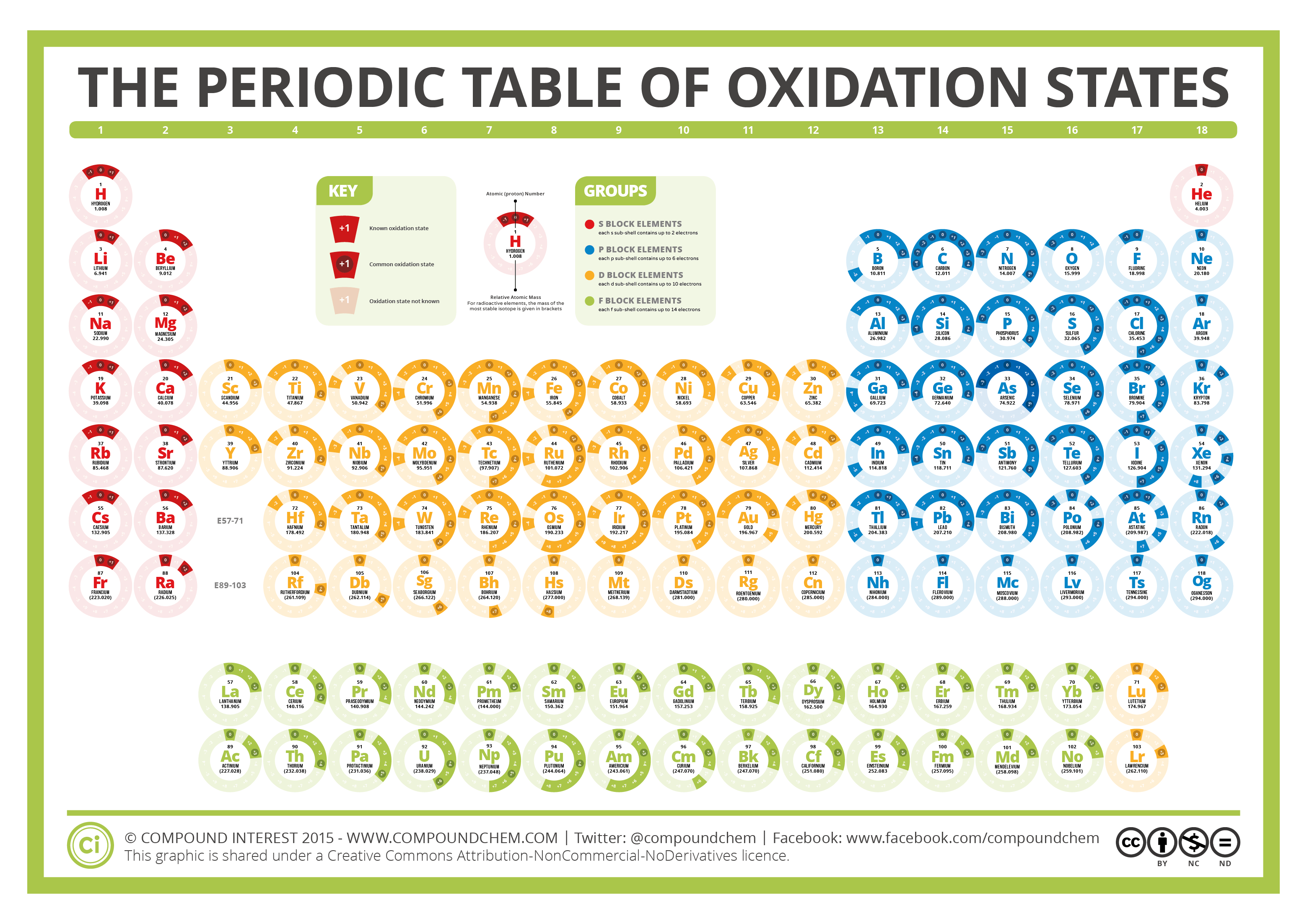
The Periodic Table of Oxidation States Compound Interest
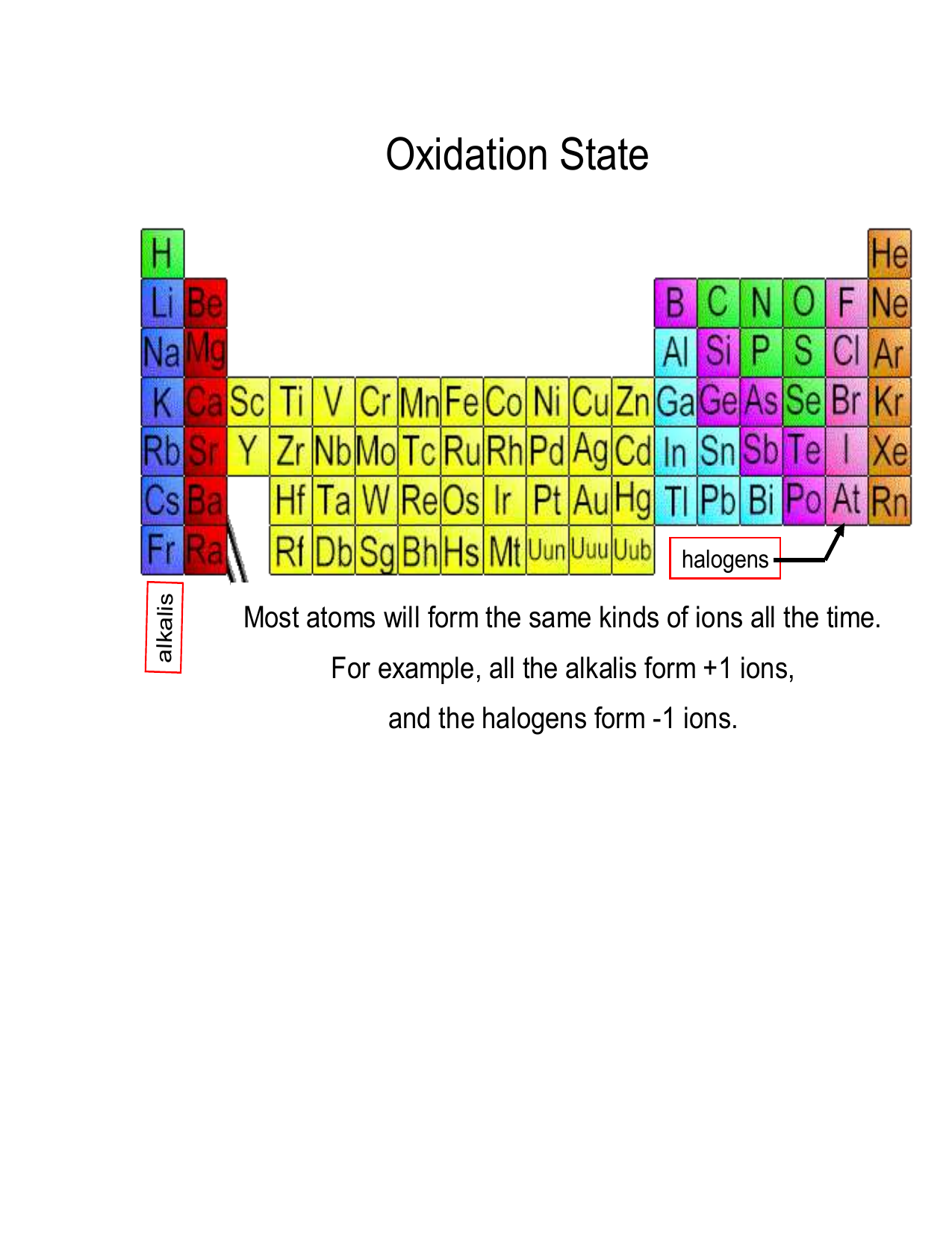
Oxidation Numbers
Web The Oxidation Number Is A Positive Or Negative Number That Is Assigned To An Atom To Indicate Its Degree Of Oxidation Or Reduction.
Redox Reactions Are Characterized By A Transfer Of Electrons.
Oxidation States Of S Block.
In Other Words, The Oxidation Number Gives The Degree Of Oxidation (Loss Of Electrons) Or Reduction (Gain Of Electrons) Of The Atom In A Compound.
Related Post: