Chart Of Hydrocarbons
Chart Of Hydrocarbons - Let's see learn how to name hydrocarbons by adding an appropriate suffix to the root word. Learn about the types, structures, and uses of hydrocarbons. Select all / deselect all. Name saturated and unsaturated hydrocarbons, and molecules derived from them. Web estimated at 511 billion barrels, the area would rank as the second largest crude oil reserve by region in the world, behind only that of the middle east, whose proven reserves stood at over 871. Name saturated and unsaturated hydrocarbons, and molecules derived from them; Alkanes, alkenes, alkynes and arenes. Ethane ( c 2 h 6. The classifications for hydrocarbons are summarized below. Web explain the importance of hydrocarbons and the reason for their diversity; Because alkane molecules are nonpolar, they are insoluble in water, which is a polar solvent, but are soluble in nonpolar and slightly polar solvents. Select all / deselect all. The hydrocarbons ethane, ethene, and ethyne provide an example of how each type of bond can affect the geometry of a molecule: The classifications for hydrocarbons are summarized below. Web the. They are broadly classified into two groups: An acyclic saturated hydrocarbon, with the general formula c n h 2n+2. Hydrocarbons are a class of organic compounds formed solely from carbon and hydrogen. Web that is because aramco is the linchpin of the strategy of muhammad bin salman, saudi arabia’s crown prince and de facto ruler, to end his country’s reliance. Learn about the types, structures, and uses of hydrocarbons. Web let’s consider their physical properties first. C 6 through c 10 alkanes, alkenes, cycloalkanes, and aromatic hydrocarbons are the main components of gasoline, naphtha, jet fuel, and specialized industrial solvent mixtures. Explain the importance of hydrocarbons and the reason for their diversity. Describe the reactions characteristic of saturated and unsaturated. Learn about the types, structures, and uses of hydrocarbons. Web explain the importance of hydrocarbons and the reason for their diversity; Name saturated and unsaturated hydrocarbons, and molecules derived from them; The four general classes of hydrocarbons are: Aromatic compounds derive their names from the fact that many of these compounds in the early days of discovery were grouped because. Aromatic compounds derive their names from the fact that many of these compounds in the early days of discovery were grouped because they were oils with fragrant odors. Web molweight, melting and boiling point, density, flash point and autoignition temperature, as well as number of carbon and hydrogen atoms in each molecule for 200 different hydrocarbons. Web the smallest alkane. Combustion (extreme oxidation) alkene + o 2 co →. Web handbook of hydrocarbons presents tables giving the most important physical properties of all hydrocarbons whose boiling points have been recorded, in such form that all compounds. The four general classes of hydrocarbons are: Ethane ( c 2 h 6. Figure 2 , by openstax college, biology ( cc by 3.0. Web molweight, melting and boiling point, density, flash point and autoignition temperature, as well as number of carbon and hydrogen atoms in each molecule for 200 different hydrocarbons. Hydrocarbons can feature simple or relatively complex structures and can be generally classified into four subcategories, namely alkanes, alkenes, alkynes, and. Learn about the types, structures, and uses of hydrocarbons. As we. Note that the gases leave at the top of the column, the liquids condense in the middle. Web explain the importance of hydrocarbons and the reason for their diversity; Identify structural and geometric isomers of hydrocarbons Image modified from carbon: Web understand some physical properties like melting points, boiling points, and solubilities of hydrocarbons. Web the smallest alkane is methane: Combustion (extreme oxidation) alkene + o 2 co →. Identify structural and geometric isomers of. Web explain the importance of hydrocarbons and the reason for their diversity; Image modified from carbon: Methane is the predominant component of natural gas. The classifications for hydrocarbons are summarized below. C 6 through c 10 alkanes, alkenes, cycloalkanes, and aromatic hydrocarbons are the main components of gasoline, naphtha, jet fuel, and specialized industrial solvent mixtures. An acyclic saturated hydrocarbon, with the general formula c n h 2n+2. It also offers a specialty products segment for. Web handbook of hydrocarbons presents tables giving the most important physical properties of all hydrocarbons whose boiling points have been recorded, in such form that all compounds. As we considered organic structures in the earlier portions of this book, alkanes were presented as examples because they are in many ways the simplest of organic molecules. Name saturated and unsaturated hydrocarbons, and molecules derived from them; To make four covalent bonds, the c atom bonds to four h atoms, making the molecular formula for methane ch 4. Name saturated and unsaturated hydrocarbons, and molecules derived from them; Web molweight, melting and boiling point, density, flash point and autoignition temperature, as well as number of carbon and hydrogen atoms in each molecule for 200 different hydrocarbons. The diagram for methane is misleading, however; Image modified from carbon: Learn about the types, structures, and uses of hydrocarbons. Describe the reactions characteristic of saturated and unsaturated hydrocarbons; Aromatic compounds derive their names from the fact that many of these compounds in the early days of discovery were grouped because they were oils with fragrant odors. Methane is the predominant component of natural gas. Identify structural and geometric isomers of. Hydrocarbons can feature simple or relatively complex structures and can be generally classified into four subcategories, namely alkanes, alkenes, alkynes, and. Describe the reactions characteristic of saturated and unsaturated hydrocarbons. Web the diagram below summarises the main fractions from crude oil and their uses and the trends in properties.
1. Classification of Hydrocarbons YouTube
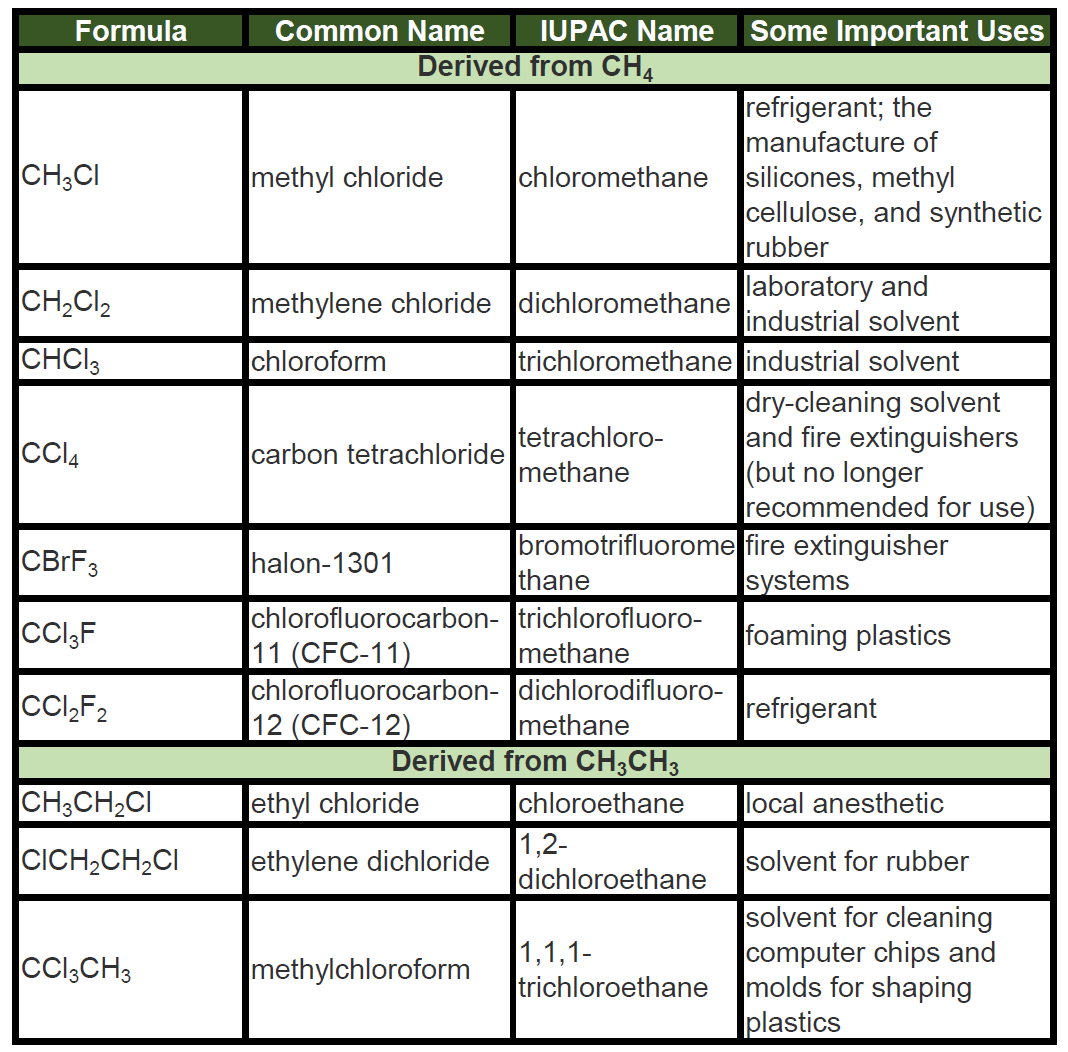
Chemical properties of hydrocarbons. Properties of Hydrocarbons. 20221024
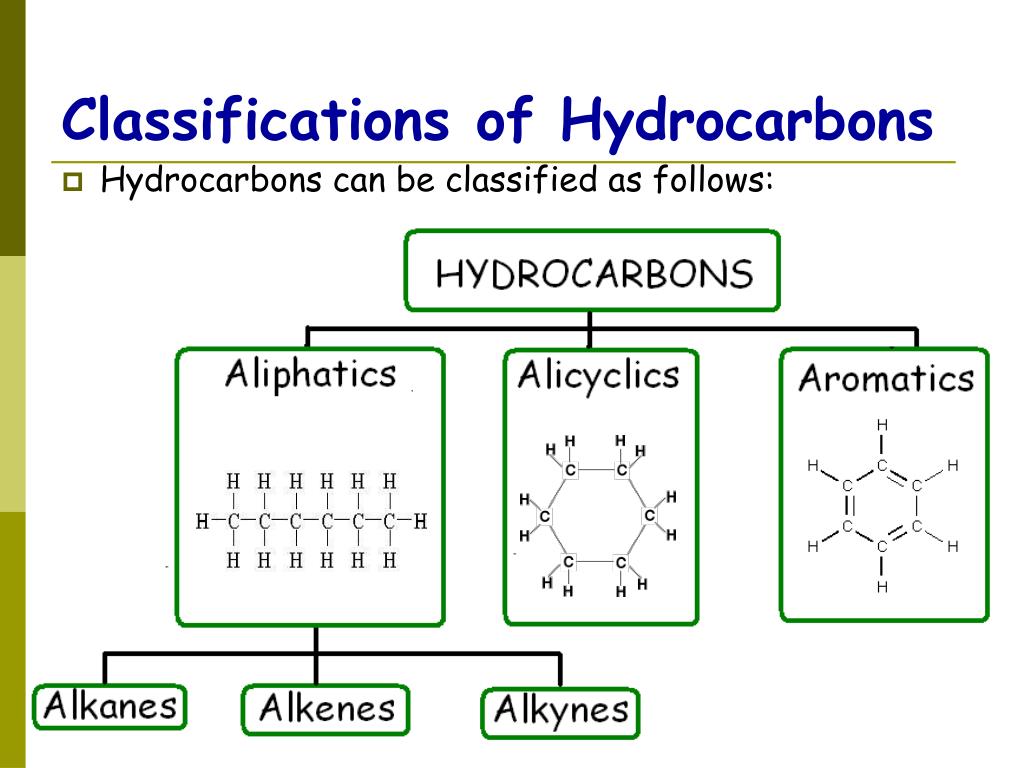
Classification Of Hydrocarbons My XXX Hot Girl

Naming Hydrocarbons Chart
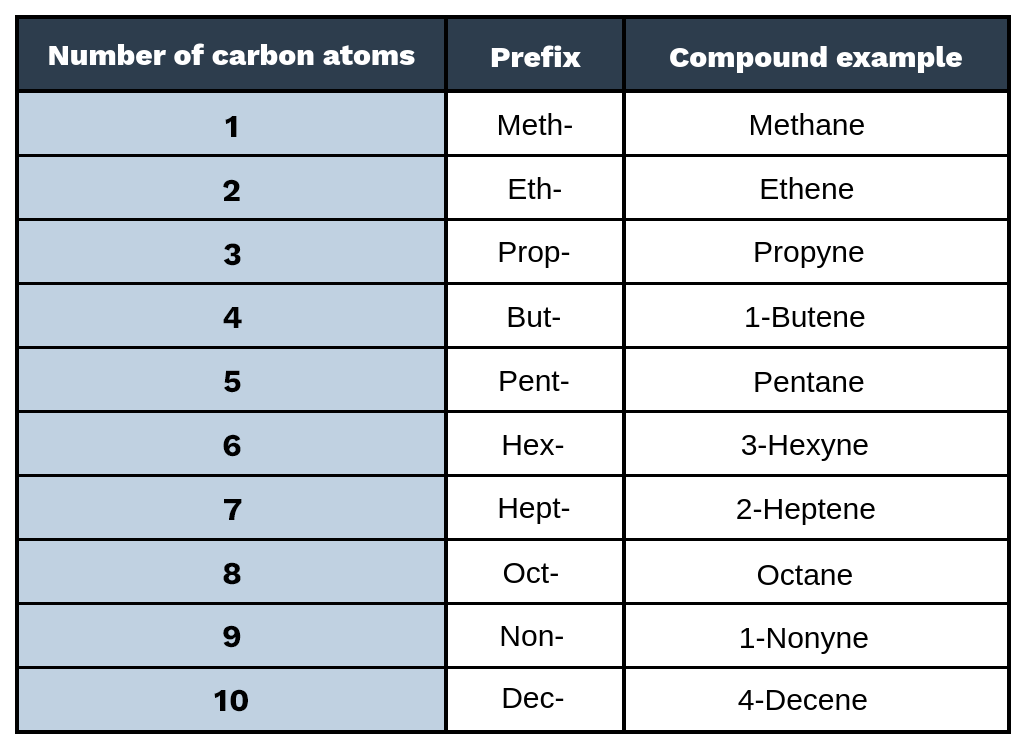
Naming Hydrocarbons Chart

Chemical Structures of Various Categories of Hydrocarbons Download

3.2. Nomenclature of unsaturated hydrocarbons Organic Chemistry 1 An
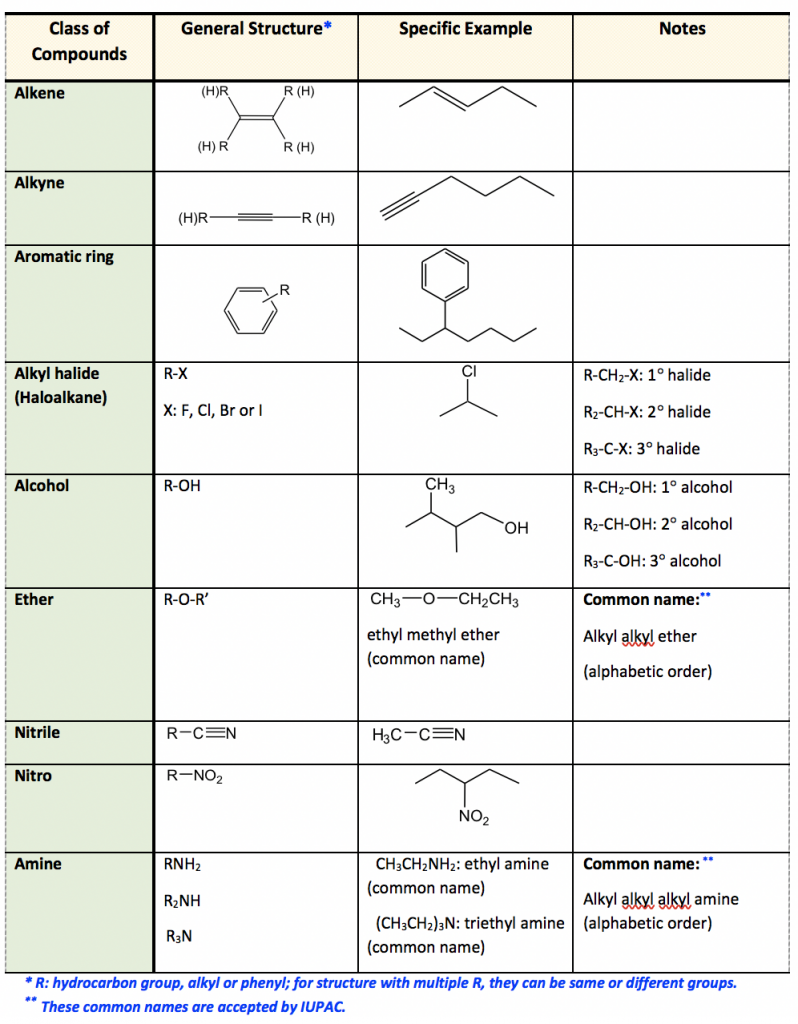
Naming Hydrocarbons Chart

Chemistry GCSE Revision Organic Chemistry

Schematic of Hydrocarbon Categories Download Scientific Diagram
Describe The Reactions Characteristic Of Saturated And Unsaturated Hydrocarbons;
Web The Four General Classes Of Hydrocarbons Are:
C 6 Through C 10 Alkanes, Alkenes, Cycloalkanes, And Aromatic Hydrocarbons Are The Main Components Of Gasoline, Naphtha, Jet Fuel, And Specialized Industrial Solvent Mixtures.
Aromatic Compounds Derive Their Names From The Fact That Many Of These Compounds In The Early Days Of Discovery Were Grouped Because They Were Oils With Fragrant Odors.
Related Post: