Atomic Radius Periodic Table Chart
Atomic Radius Periodic Table Chart - Web explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. This is a periodic table with atomic radius values mentioned on it. For instance, the radii generally decrease rightward along each period (row) of the table, from the alkali metals to the noble gases; Web this table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. Web select from the following links to see visual periodicity representations for atomic radii, covalent radii, and van der waals radii. What is atomic radius on a periodic table? Web interactive periodic table showing names, electrons, and oxidation states. Web atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its relationship to other atomic properties such as nuclear charge and shielding. Web atomic radius trends on periodic table (video) | khan academy. Web the periodic table contains nist’s latest critically evaluated data for atomic properties of the elements. Web atomic radius of all the elements are mentioned in the chart below. Web this table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. The values are in picometres (pm). The atomic radius of atoms generally increases from top to bottom within a group. Web when you move. The values are in picometres (pm). Below mentioned radii are the van der waals radius in picometer (pm)). For instance, the radii generally decrease rightward along each period (row) of the table, from the alkali metals to the noble gases; For isolated neutral atoms, the atomic nucleus ranges from 30 picometers (trillionths of a meter) and 300 pm. As shown. Web the trend on a graph. The atomic radius is the distance from the center of the nucleus to the outermost orbit of an atom. Web major periodic trends include: Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. Web the atomic radius is the size of the atom, typically measured by the distance from the nucleus. Web atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its relationship to other atomic properties such as nuclear charge and shielding. Atomic radii (empirical) atomic radii (absolute) atomic radii (density cutoff) covalent radii revisited (2008 values) covalent radii (molecular. What is atomic radius on a periodic table? This is a periodic table with atomic radius values mentioned on it. The atomic radius of atoms generally increases from top to bottom within a group. The atomic radius is the distance from the center of the nucleus to the outermost orbit of an atom. Web major periodic trends include: How atomic radius is defined, and trends across a period and down a group. Web when you move right across a period, you’re adding more protons and electrons, but you stay at the same number of shells, rather than forcing electrons into higher shells, allowing the magnetic attraction between the protons and electrons to keep the radius of the atom. Web this table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. Francium has the largest atomic size on the periodic table, and helium has the smallest atomic size. Web the trend on a graph. Web when you move right across a period, you’re adding more protons and electrons,. As shown in the graph below, the atomic radius is largest at the first element in each period, and it decreases down each period. Web the atomic radius is the size of the atom, typically measured by the distance from the nucleus of the atom to the electron clouds around the nucleus. What is atomic radius on a periodic table?. Ionic radii are also available. The values are in picometres (pm). Web atomic radius (ionic radius) atomic radius is the distance from the nucleus to the outermost stable electron while ionic radius is half the distance between two atomic nuclei that are just touching each other. Web atomic radii vary in a predictable and explicable manner across the periodic table.. For instance, the radii generally decrease rightward along each period (row) of the table, from the alkali metals to the noble gases; Web the atomic radius is the average distance from the center of the nucleus of a neutral atom to the outer boundary of its electron shell. Web explore how atomic radius changes with atomic number in the periodic. Web atomic radius (ionic radius) atomic radius is the distance from the nucleus to the outermost stable electron while ionic radius is half the distance between two atomic nuclei that are just touching each other. Web explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. Below mentioned radii are the van der waals radius in picometer (pm)). Web atomic radius trends on periodic table (video) | khan academy. The atomic radius of atoms generally increases from top to bottom within a group. Ionic radii are also available. Web the periodic table contains nist’s latest critically evaluated data for atomic properties of the elements. This is due to the way electrons form shells around the nucleus. Web the trend on a graph. Web major periodic trends include: Atomic radii (empirical) atomic radii (absolute) atomic radii (density cutoff) covalent radii revisited (2008 values) covalent radii (molecular single bond) Web atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. For isolated neutral atoms, the atomic nucleus ranges from 30 picometers (trillionths of a meter) and 300 pm. Web when you move right across a period, you’re adding more protons and electrons, but you stay at the same number of shells, rather than forcing electrons into higher shells, allowing the magnetic attraction between the protons and electrons to keep the radius of the atom smaller. The following table shows atomic radii computed from theoretical models, as published by enrico clementi and others in 1967. Visualize trends, 3d orbitals, isotopes, and mix compounds.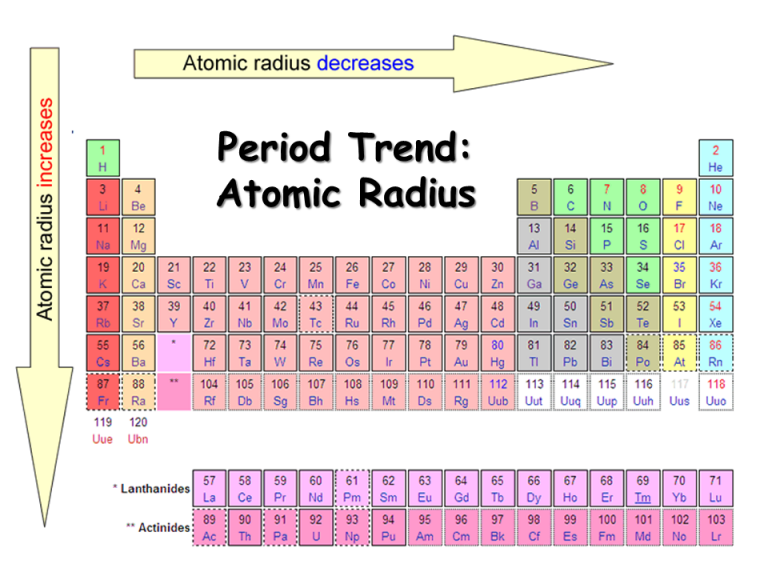
Atomic Radius NEETLab
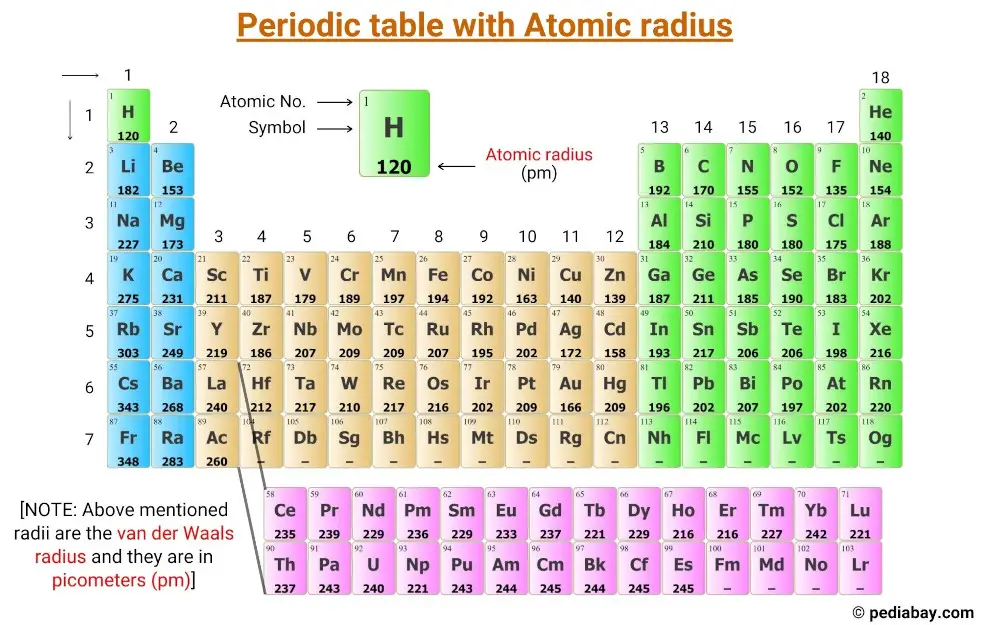
Atomic Radius of Elements (With Periodic table Chart) Pediabay

Atomic Radius of Elements
Atomic radius chart mindsstorm
Atomic Radius Trends of the Periodic Table
.png)
CK12Foundation
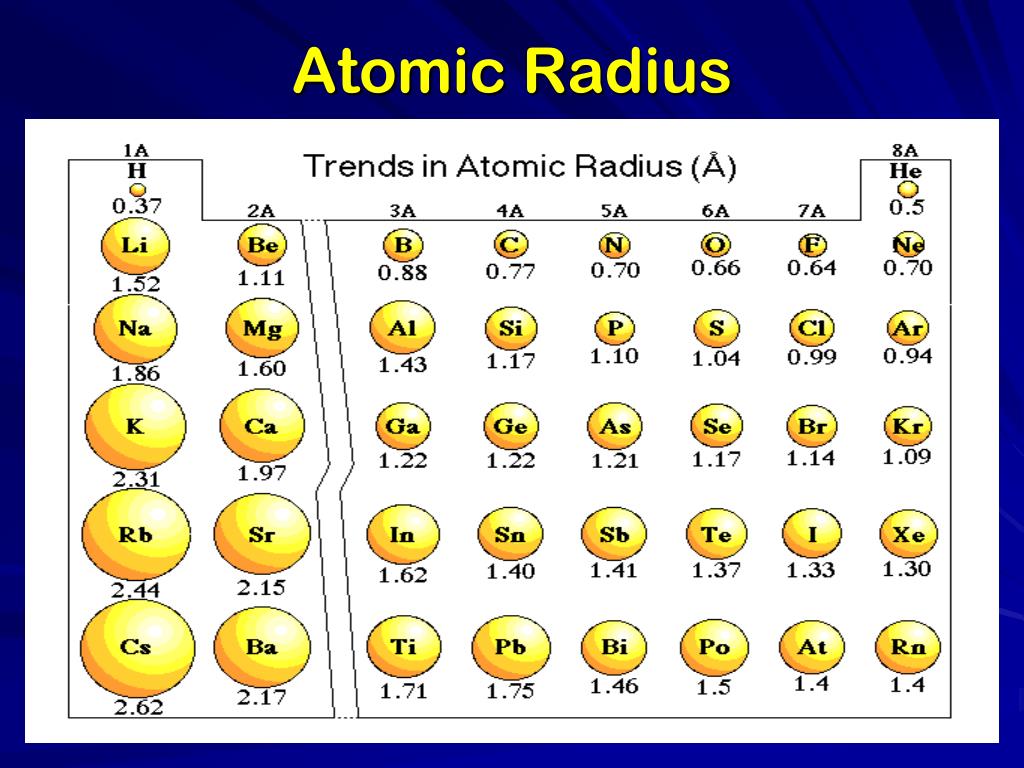
PPT Periodic Table PowerPoint Presentation, free download ID2617702
/accurate-illustration-of-the-periodic-table-82020791-57cc76f23df78c71b66efbd7.jpg)
Atomic Radius Table Pdf Elcho Table

Atomic Radius of Elements The Periodic Table
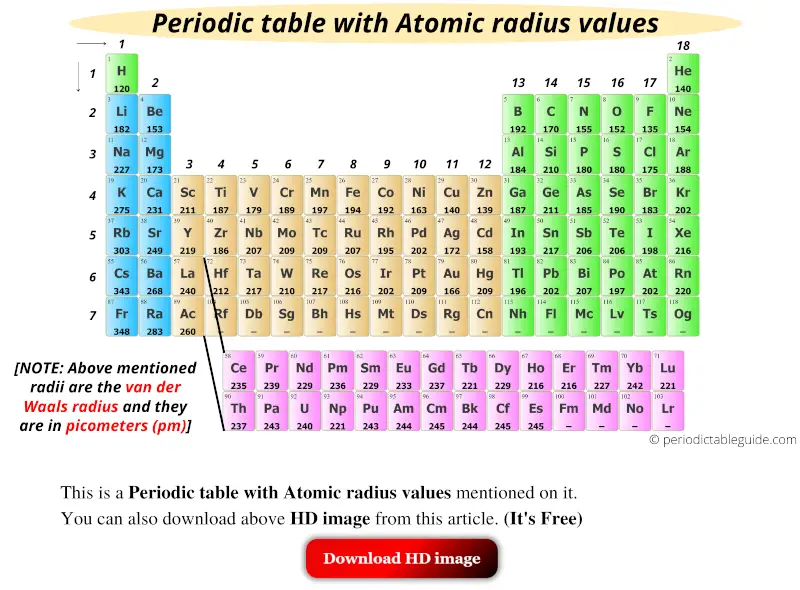
Get the Periodic table with Atomic radius values (Img+Chart)
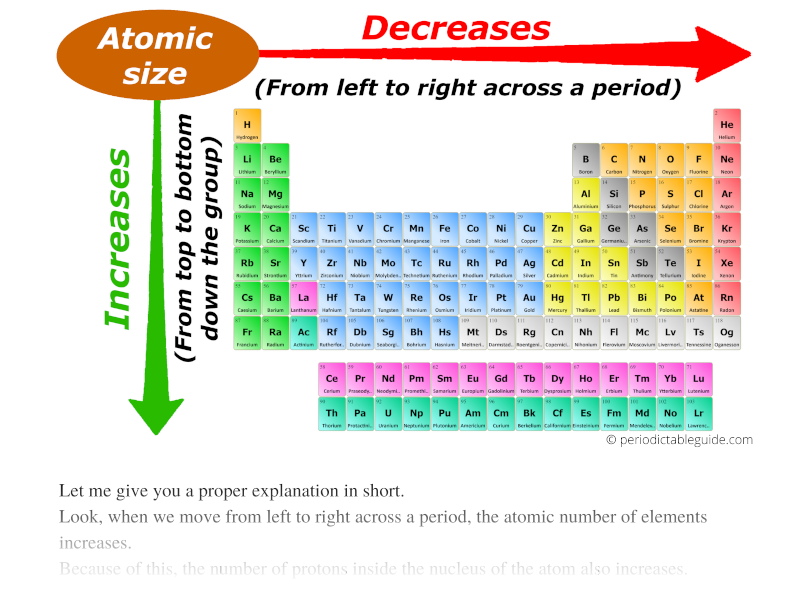
For Instance, The Radii Generally Decrease Rightward Along Each Period (Row) Of The Table, From The Alkali Metals To The Noble Gases;
Web In The Periodic Table, Atomic Radii Decrease From Left To Right Across A Row And Increase From Top To Bottom Down A Column.
These Related Values Display The Same Trend In The Periodic Table.
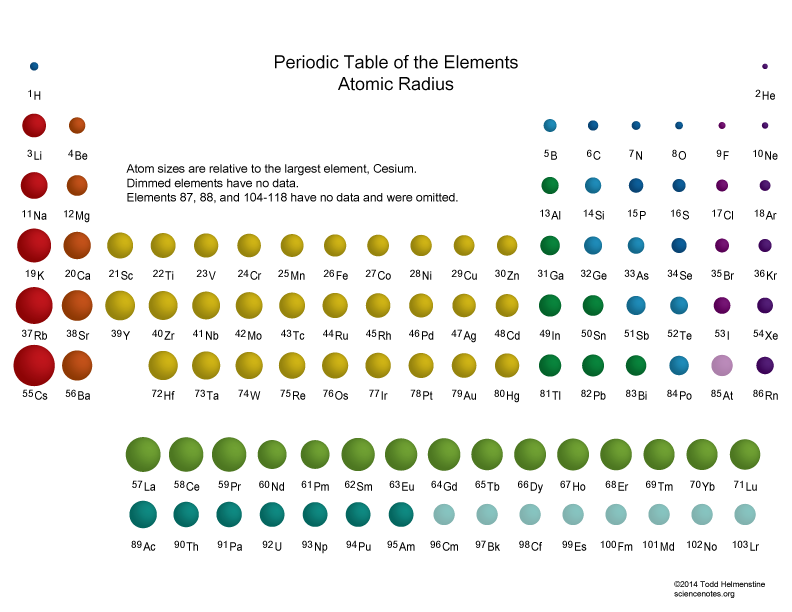
Francium Has The Largest Atomic Size On The Periodic Table, And Helium Has The Smallest Atomic Size.
Related Post: