Atom Size Chart
Atom Size Chart - However, some atoms are larger or smaller than others, and this influences their chemistry. Scientists’ ideas about atoms have changed over time. It includes element names, symbols, groups, atomic numbers, and atomic masses. A convenient unit of length for measuring atomic sizes is the angstrom (å), defined as 10 −10 metre. Web the atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Web this periodic table chart shows the relative sizes of each element. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). Below mentioned radii are the van der waals radius in picometer (pm)). Web all atoms are roughly the same size, whether they have 3 or 90 electrons. How does atomic size increase on periodic table? Web the atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Four widely used definitions of. You can download a pdf version of the table for printing. Look up chemical element names, symbols, atomic masses and other. In each case, the negative ion is much larger than the atom from which it was formed. Atomic radius in the periodic table. Below mentioned radii are the van der waals radius in picometer (pm)). Scientists’ ideas about atoms have changed over time. Web atomic radius of all the elements are mentioned in the chart below. In this section, we discuss how atomic and ion “sizes” are defined and obtained. Web interactive periodic table showing names, electrons, and oxidation states. Each atom's size is scaled to the largest element, cesium to show the trend of atom size. We know it has a posoitively charged center (the nucleus), which is surounded by mving electrosn. Each atom is. In fact, the negative ion can be more than twice as large as the neutral atom. Below mentioned radii are the van der waals radius in picometer (pm)). [ pdf file] jpg and png periodic tables. Atomic size & atomic radius. Web all atoms are roughly the same size, whether they have 3 or 90 electrons. Web the size and shape of molecules are as much a part of molecular structure as is the order in which the component atoms are bonded. Web all atoms are roughly the same size, whether they have 3 or 90 electrons. In this section, we discuss how atomic and ion “sizes” are defined and obtained. Web how is atomic size. Each atom's size is scaled to the largest element, cesium to show the trend of atom size. In each case, the negative ion is much larger than the atom from which it was formed. Web what does it mean to measure the “size” of an atom? You can download a pdf version of the table for printing. Web all atoms. [ pdf file] jpg and png periodic tables. Web interactive periodic table showing names, electrons, and oxidation states. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! How does atomic size increase on periodic table? Web the atomic radius of a chemical element is. Web the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Web atomic size changes in predictable ways as one moves around the periodic table. Web the size and shape of molecules are as much a part of molecular structure as is the order in which the. Web interactive periodic table showing names, electrons, and oxidation states. Png files, in particular, are very small file sizes that print very clearly. Web this special periodic table shows the relative size of atoms of periodic table elements based on atomic radius data. Visualize trends, 3d orbitals, isotopes, and mix compounds. Web all atoms are roughly the same size, whether. An atom is not like a billiard ball. However, some atoms are larger or smaller than others, and this influences their chemistry. Web atomic radius of all the elements are mentioned in the chart below. Web interactive periodic table showing names, electrons, and oxidation states. Each atom is shown relative to the largest atom, cesium. Web a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an isoelectronic series, shows a clear correlation between increasing nuclear charge and decreasing size. Below mentioned radii are the van der waals radius in picometer (pm)). Web the size and shape of molecules are as much a part of molecular structure as is the order in which the component atoms are bonded. Web interactive periodic table showing names, electrons, and oxidation states. It features our favorite color scheme of all the tables we’ve made. In each case, the negative ion is much larger than the atom from which it was formed. Web the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Png files, in particular, are very small file sizes that print very clearly. Web this 118 element periodic table is a 1920×1080 hd wallpaper. By anne marie helmenstine, ph.d. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). Classification of elements and periodicity in properties. Web all atoms are roughly the same size, whether they have 3 or 90 electrons. Web the atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Web how is atomic size measured? Scientists’ ideas about atoms have changed over time.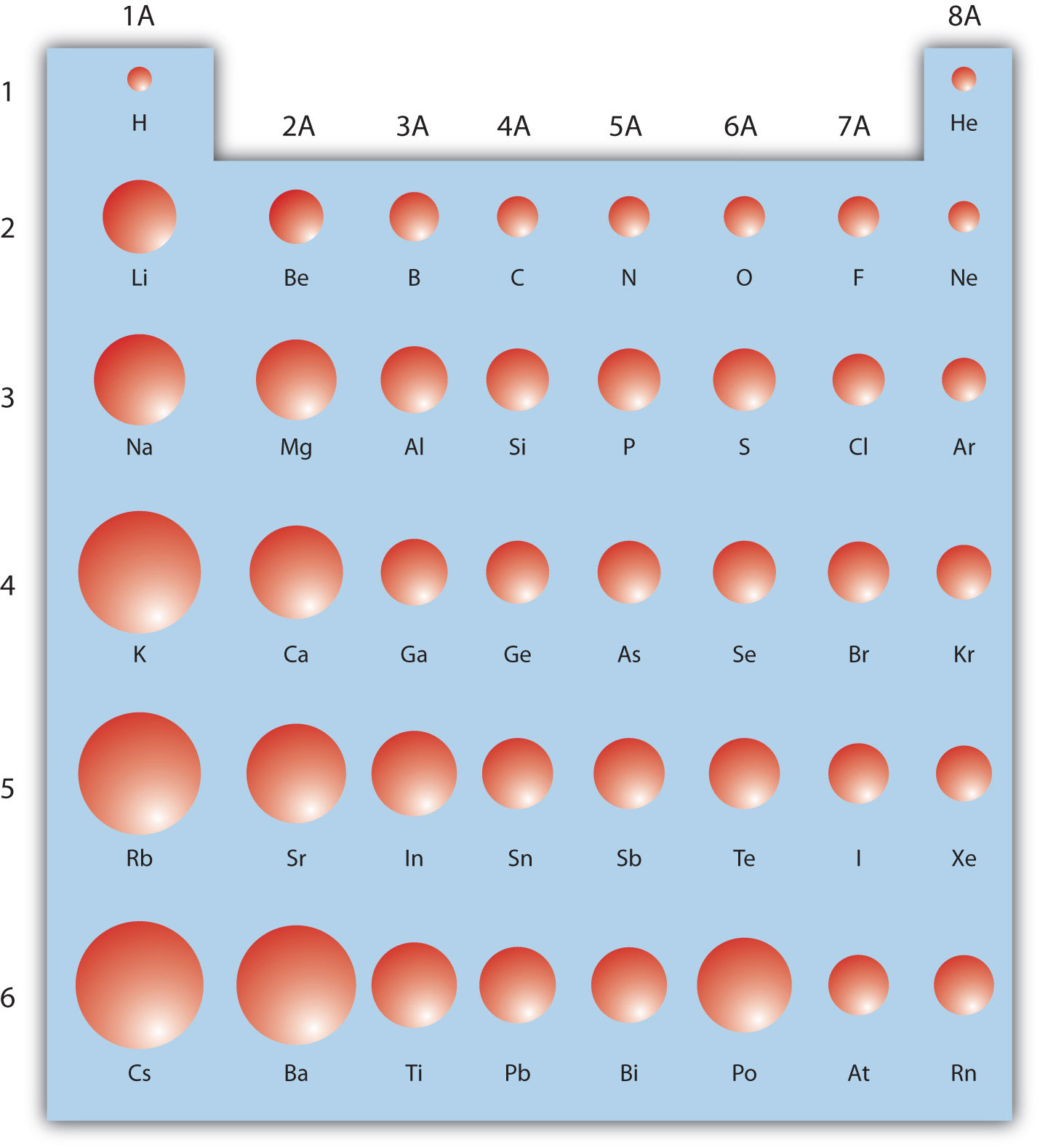
The Periodic Table
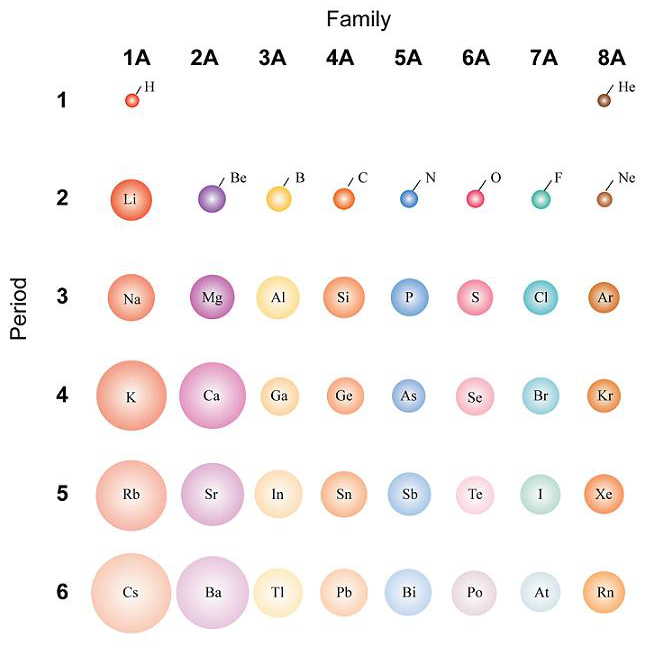
Atomic Size Introduction to Chemistry
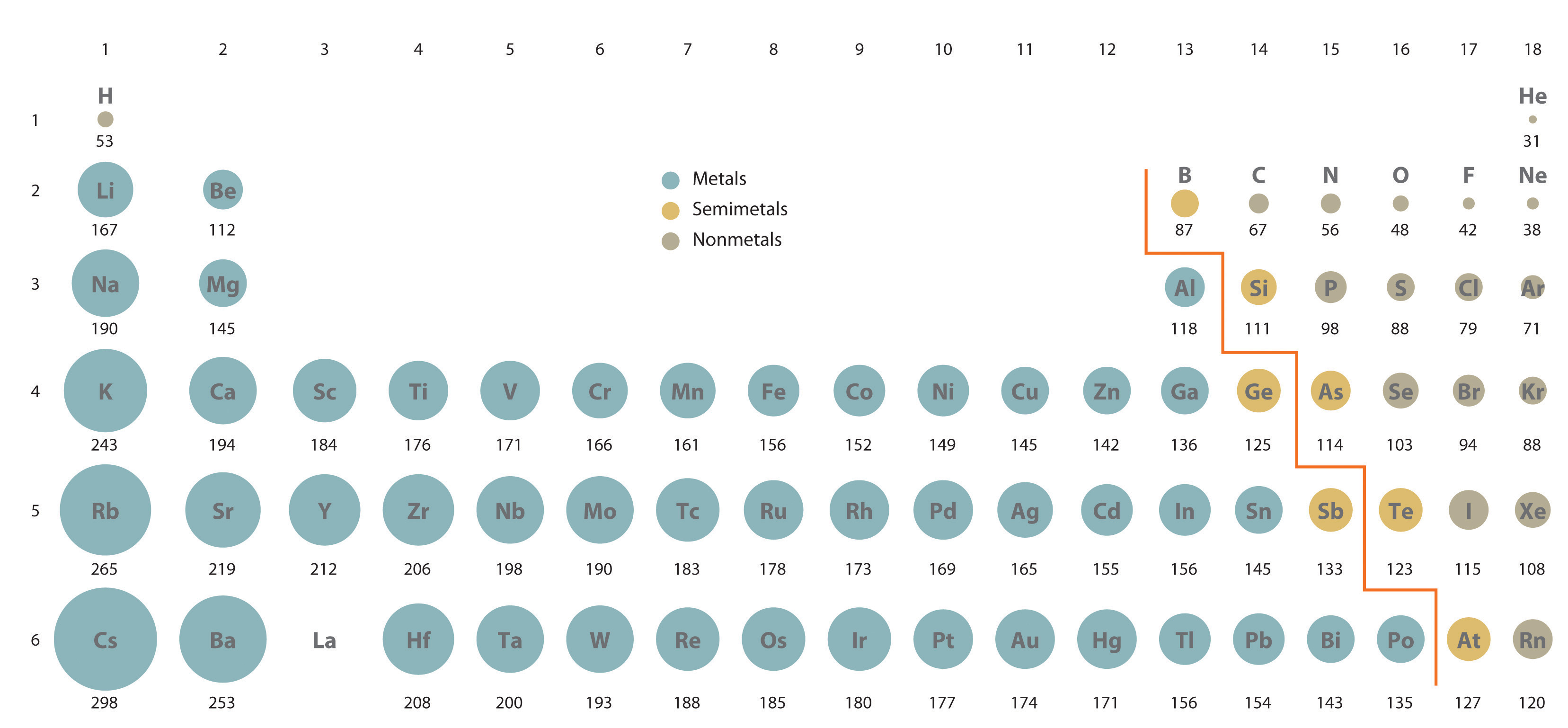
Sizes of Atoms and Ions

Atomic Sizes and Radii Charts for Chemistry
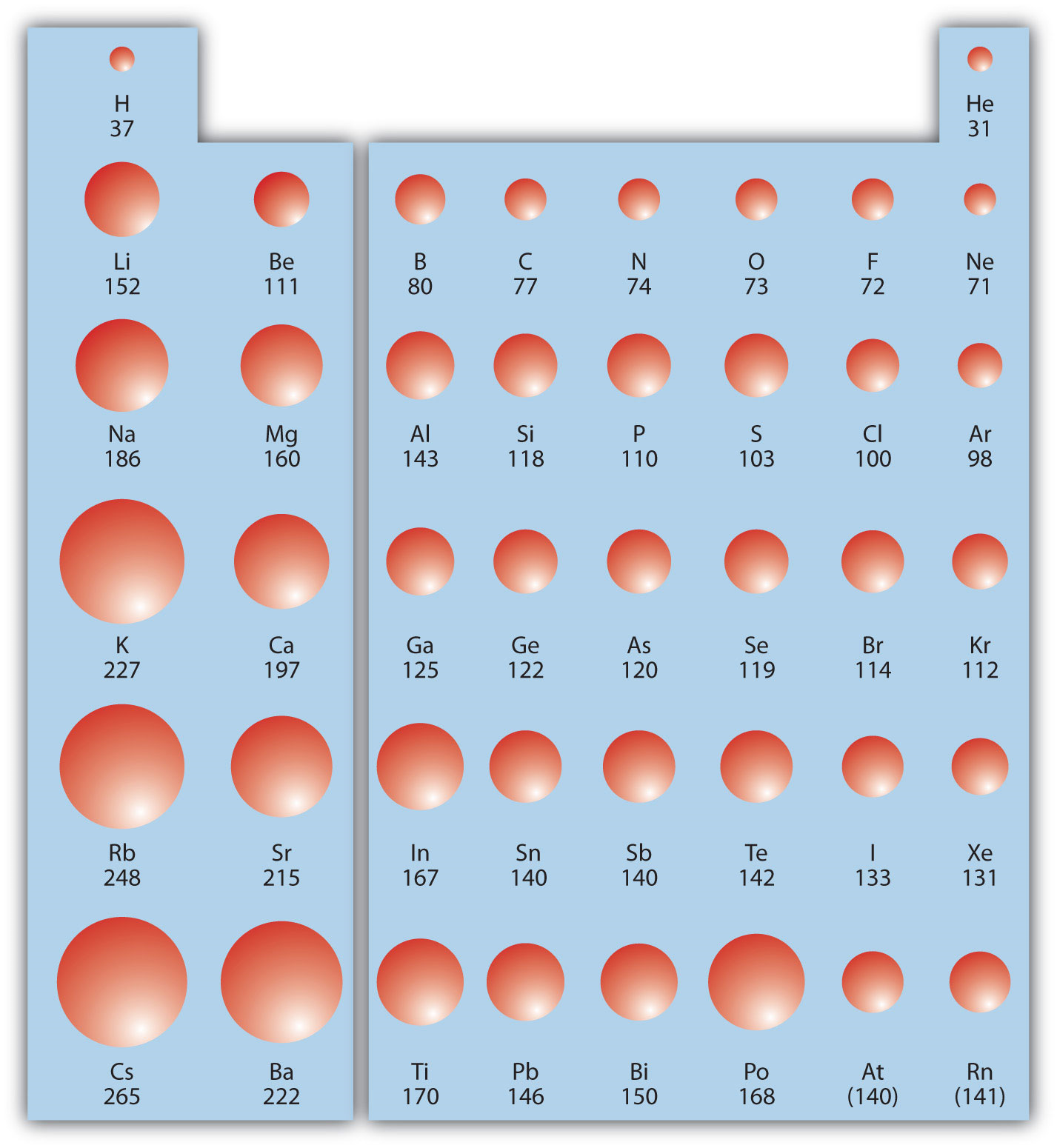
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic
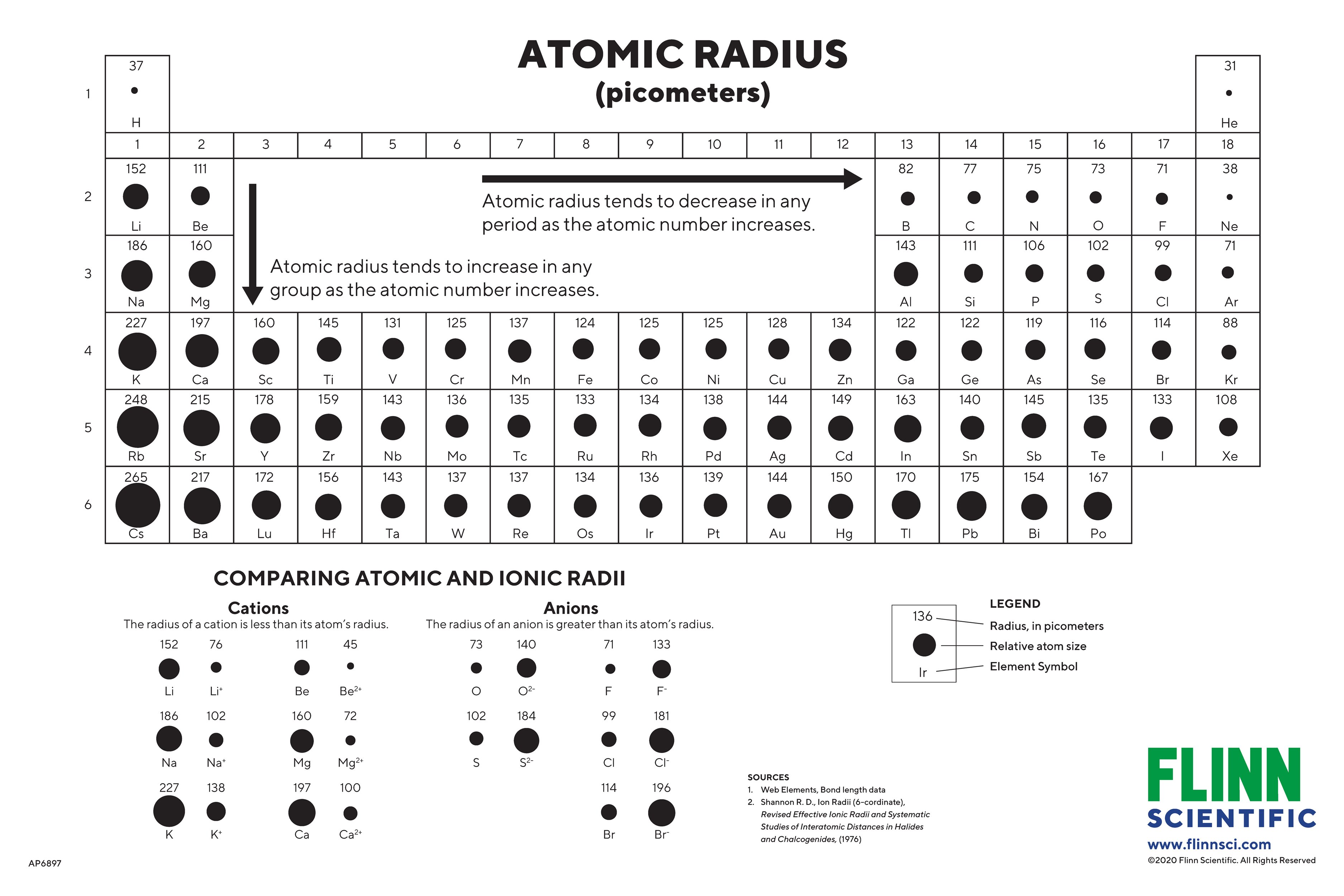
Atomic Sizes and Radii Charts for Chemistry
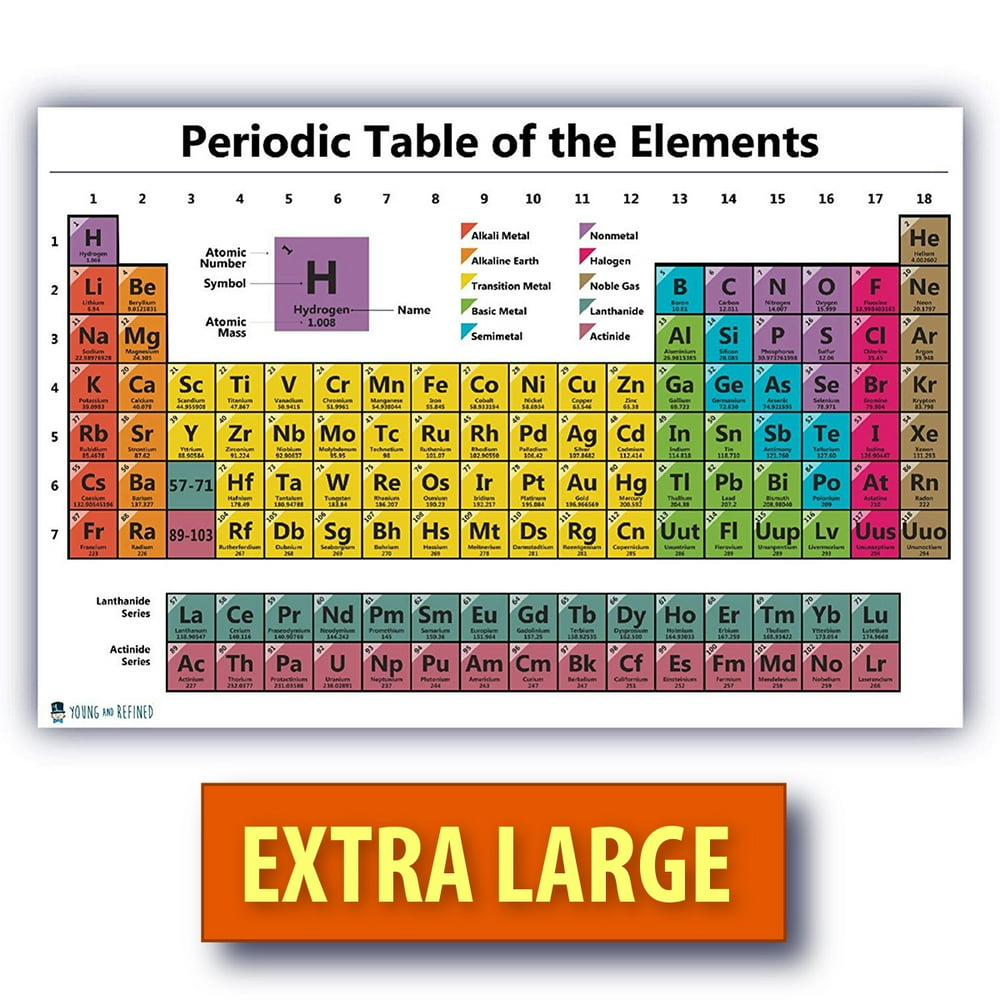
Atomic Size Chart Periodic Table
Periodic Trends in Atomic Size CK12 Foundation

This periodic table chart shows the relative sizes of each element
Periodic Trends in Atomic Size CK12 Foundation
In This Section, We Discuss How Atomic And Ion “Sizes” Are Defined And Obtained.
How Does Atomic Size Increase On Periodic Table?
Tables In Jpg And Png Format Are Image Files.
Each Atom's Size Is Scaled To The Largest Element, Cesium To Show The Trend Of Atom Size.
Related Post: