Amino Acids Chart With Pka
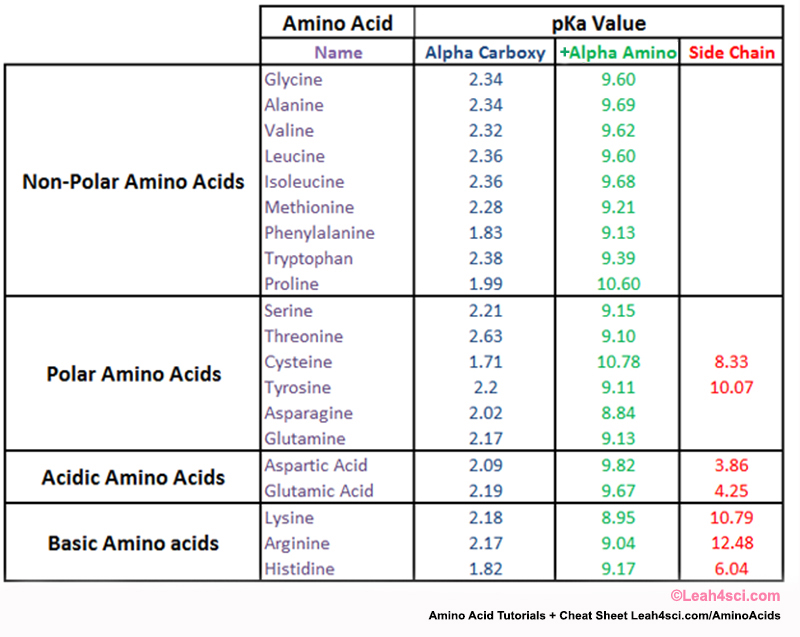
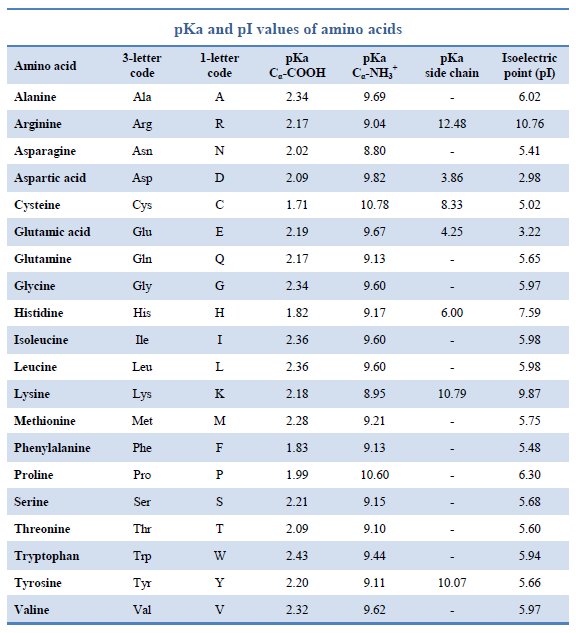
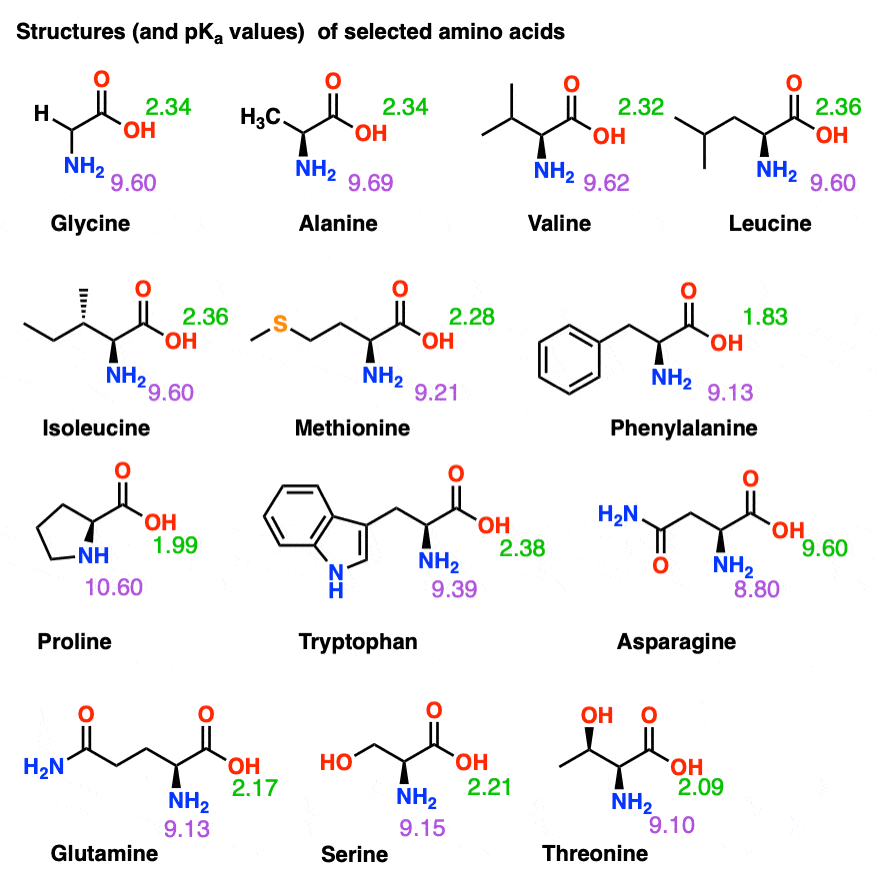
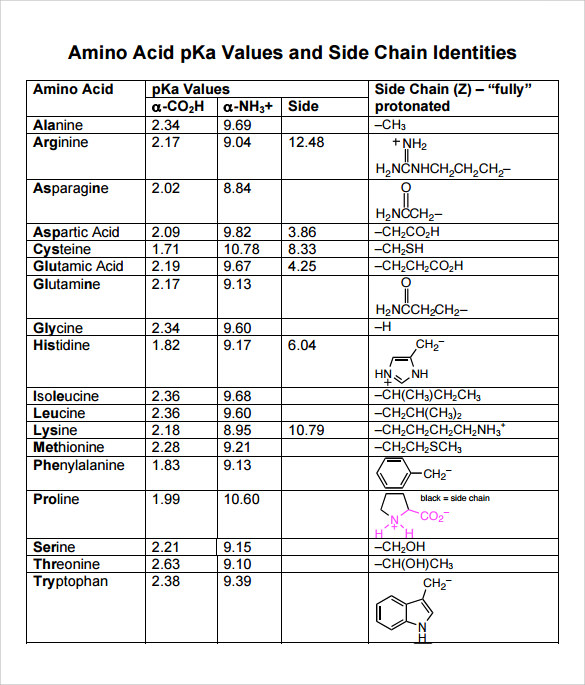
Amino Acids Chart With Pka - You will learn how to calculate the isoelectric point, and the effects of ph on the amino acid's overall charge. Web each amino acid has its own pi value based on the properties of the amino acid. Two cysteines close in space may form disulfide bridges under oxidizing conditions, prolines tend to introduce kinks in polypeptides and are often found at. Web all amino acids have the same basic structure, which is shown in figure 2.1. Web pka of all 20 amino acids: The r group side chains may be either nonpolar, polar and uncharged, or charged, depending on the functional group, the ph, and the pka of any ionizable group in the side chain. At ph values above or below the isoelectric point, the molecule will have a net charge which depends on its pi value as well as the ph of the solution in which the amino acid is found. For the four amino acids with either a strongly or weakly acidic side chain, p i is the average of the two lowest p ka values. Web amino acids are the building blocks of proteins. Created by tracy kim kovach. Web calculating isoelectric point pi from pka values. Web the isoelectric point of an amino acid is the ph at which the amino acid has a neutral charge. Pi calculations for amino acids with basic side chains. We will also discuss zwitterions, or the forms of amino acids that dominate at the isoelectric point. Two cysteines close in space may. Amino acids are the building blocks that form polypeptides and ultimately proteins. Web you should be able to classify all the amino acids by polarity, charge, aliphatic vs aromatic, and probably learn the structures and functional groups of the special amino acids (for example: 3 pkx is the negative of the logarithm of the dissociation constant for any other group. At neutral ph the amino group is protonated, and the carboxyl group is deprotonated. Web all amino acids have the same basic structure, which is shown in figure 2.1. Conjugate acid of − nh 2, i.e., − nh + 3 has pk a ~10. Web you should be able to classify all the amino acids by polarity, charge, aliphatic vs. Web each amino acid has its own pi value based on the properties of the amino acid. Web you should be able to classify all the amino acids by polarity, charge, aliphatic vs aromatic, and probably learn the structures and functional groups of the special amino acids (for example: At ph values above or below the isoelectric point, the molecule. 3 pkx is the negative of the logarithm of the dissociation constant for any other group in the molecule. That is a daunting task for 20 amino acids. Web the isoelectric point of an amino acid is the ph at which the amino acid has a neutral charge. Two cysteines close in space may form disulfide bridges under oxidizing conditions,. A homogeneous substance, something that your diet should contain in. Web amino acids are the building blocks of proteins. Most amino acids have a chiral carbon, which allows them to rotate polarized light. We will also discuss zwitterions, or the forms of amino acids that dominate at the isoelectric point. Web the isoelectric point of an amino acid is the. (advanced) references and further reading. We will also discuss zwitterions, or the forms of amino acids that dominate at the isoelectric point. Web for the 13 amino acids with a neutral side chain, p i is the average of p ka1 and p ka2. Most amino acids have a chiral carbon, which allows them to rotate polarized light. Web amino. Web all amino acids have the same basic structure, shown in figure 2.1. Properties of common amino acids. The r group side chains may be either nonpolar, polar and uncharged, or charged, depending on the functional group, the ph, and the pka of any ionizable group in the side chain. Web the pka is a measure of the strength of. Consequently, they are fundamental components of our bodies and vital for physiological functions such as protein synthesis, tissue repair and nutrient absorption. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. Pi calculations for amino acids with basic side chains. Web 20 amino acids and their functions, structures, names, properties,. Web amino acids are the building blocks of proteins. Two cysteines close in space may form disulfide bridges under oxidizing conditions, prolines tend to introduce kinks in polypeptides and are often found at. Web table of pka and pi values. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. You. Web the isoelectric point of an amino acid is the ph at which the amino acid has a neutral charge. Conjugate acid of − nh 2, i.e., − nh + 3 has pk a ~10. Properties of common amino acids. At ph values above or below the isoelectric point, the molecule will have a net charge which depends on its pi value as well as the ph of the solution in which the amino acid is found. Web calculating isoelectric point pi from pka values. For the three amino acids with a basic side chain, p i is the average of the two highest p ka values. Amino acids contain a carboxylic acid and an amino group. The isoelectric points range from 5.5 to 6.2. Web you should be able to classify all the amino acids by polarity, charge, aliphatic vs aromatic, and probably learn the structures and functional groups of the special amino acids (for example: Web all amino acids have the same basic structure, which is shown in figure 2.1. A zwitterion, also known as a dipolar ion, is a molecule or ion that has both a positive and a negative charge within its structure, resulting in overall electrical neutrality. Web for the 13 amino acids with a neutral side chain, p i is the average of p ka1 and p ka2. For the four amino acids with either a strongly or weakly acidic side chain, p i is the average of the two lowest p ka values. Web pka of all 20 amino acids: Web amino acids are the building blocks of proteins. They contain an amino group, carboxylic acid group, alpha carbon, and side chain.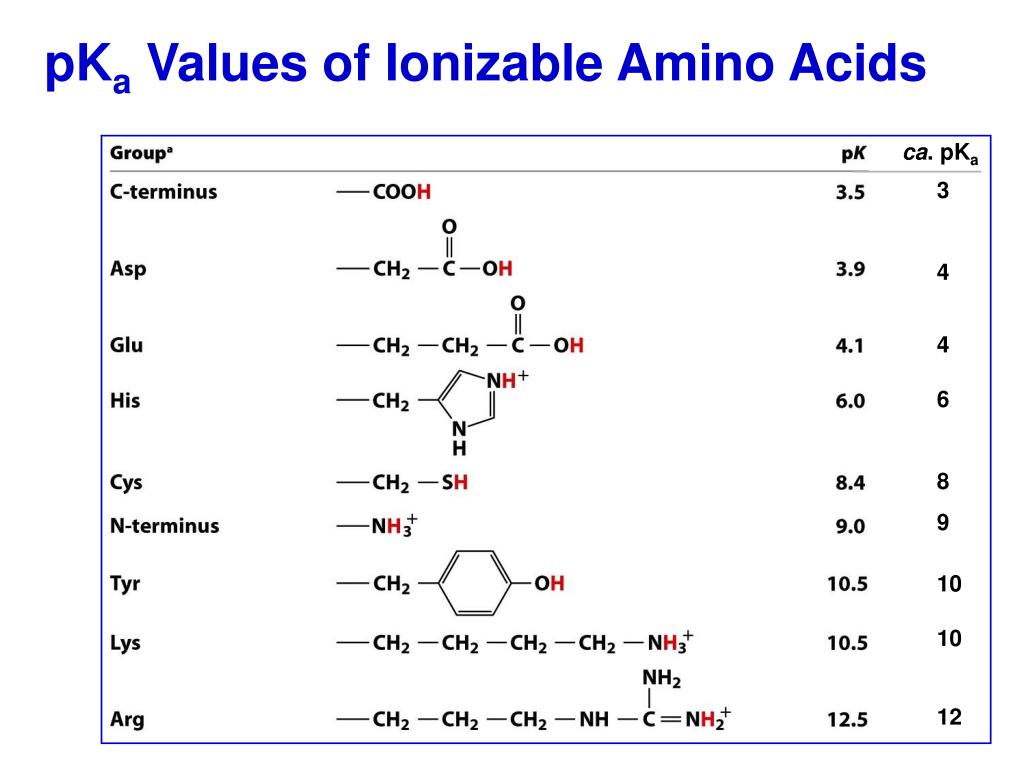
Amino Acids Pka Chart
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial
![[Infographic] Comprehensive pKa Chart r/chemistry](https://external-preview.redd.it/K3Snfd3HbLKkQbUsJ8g5GMVBN8te4Altg0_bder8QLE.jpg?auto=webp&s=d6f9b865541ac9cf9c578f5146e380a014396caa)
[Infographic] Comprehensive pKa Chart r/chemistry
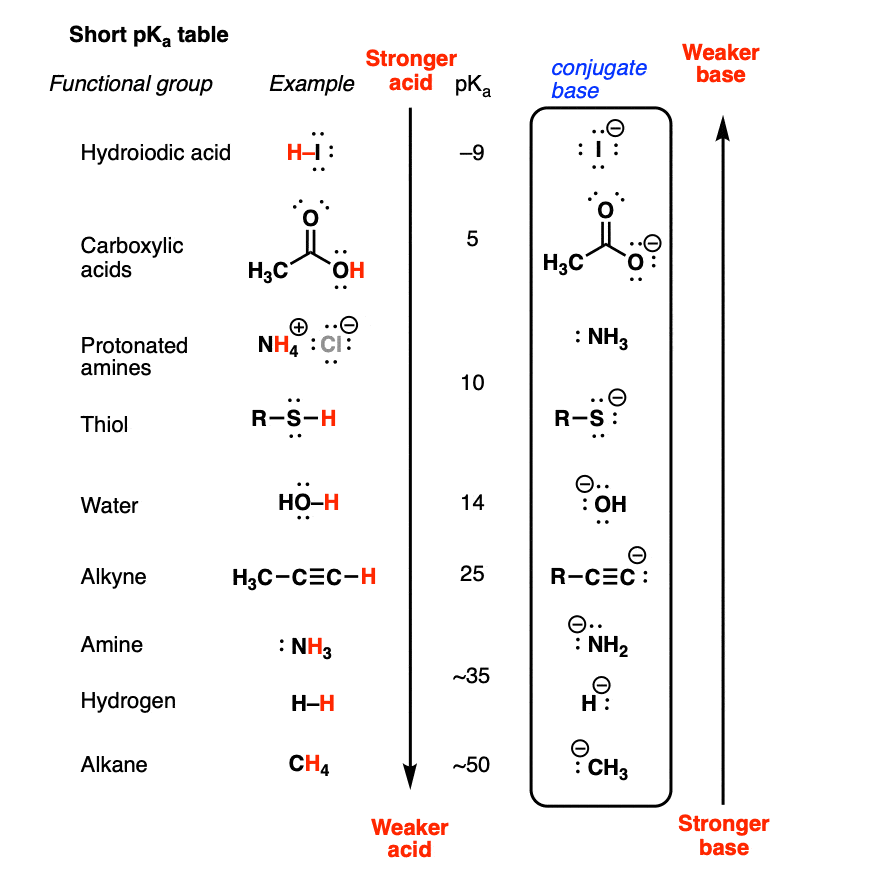
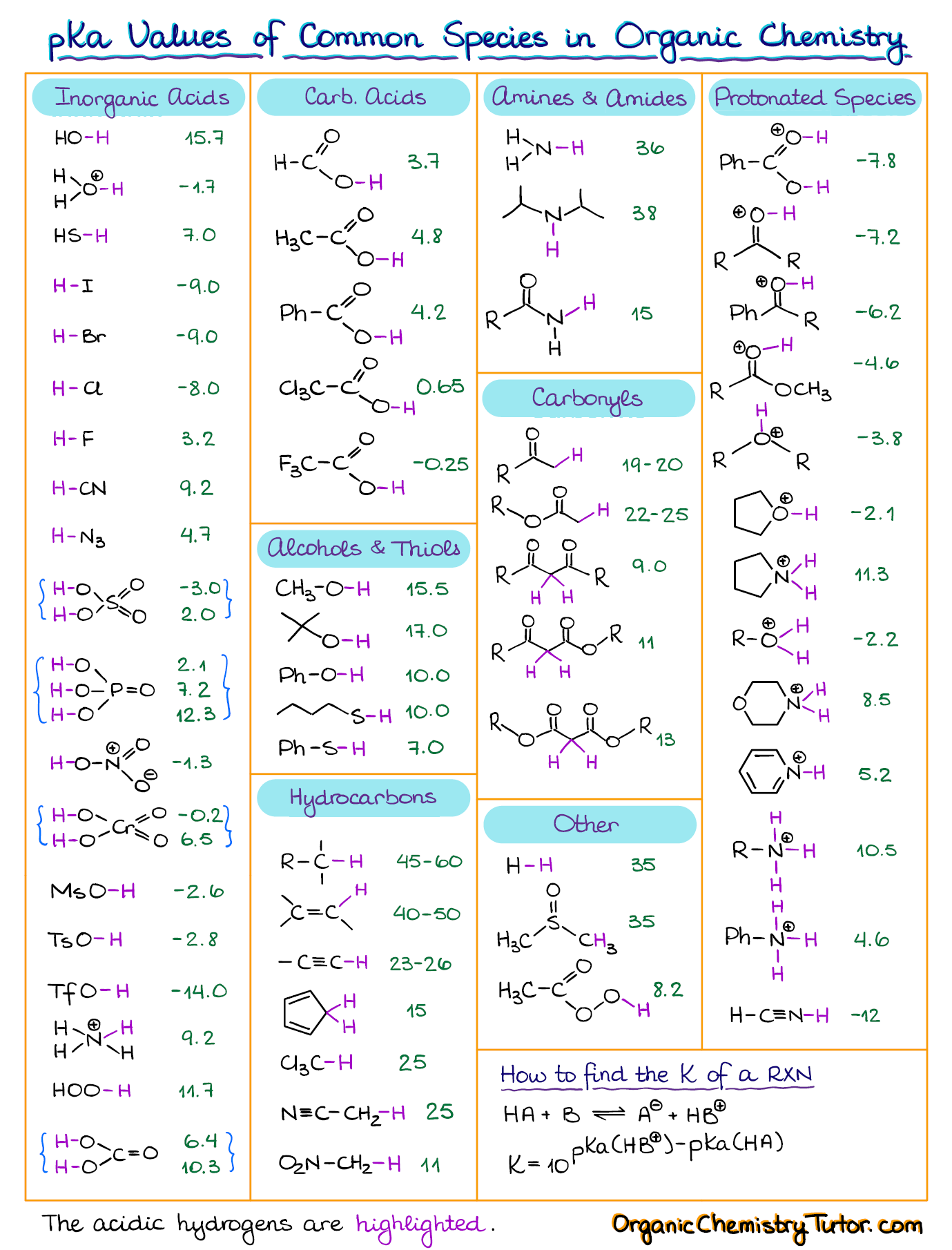
How To Use a pKa Table
Amino acid properties
Isoelectric Points of Amino Acids (and How To Calculate Them) Master
AcidBase Equilibrium Part 1 How to Use the pKa Table (2023)

Chapter 2 Protein Structure Chemistry

The pKa in Organic Chemistry Chemistry Steps
Pka Chart Amino Acids
Web 20 Amino Acids And Their Functions, Structures, Names, Properties, Classifications.
We Will Also Discuss Zwitterions, Or The Forms Of Amino Acids That Dominate At The Isoelectric Point.
The R Group Side Chains May Be Either Nonpolar, Polar And Uncharged, Or Charged, Depending On The Functional Group, The Ph, And The Pka Of Any Ionizable Group In The Side Chain.
Web Most Biochemistry Courses Will Require You To Know The Following:
Related Post: