Acid Base Extraction Flow Chart
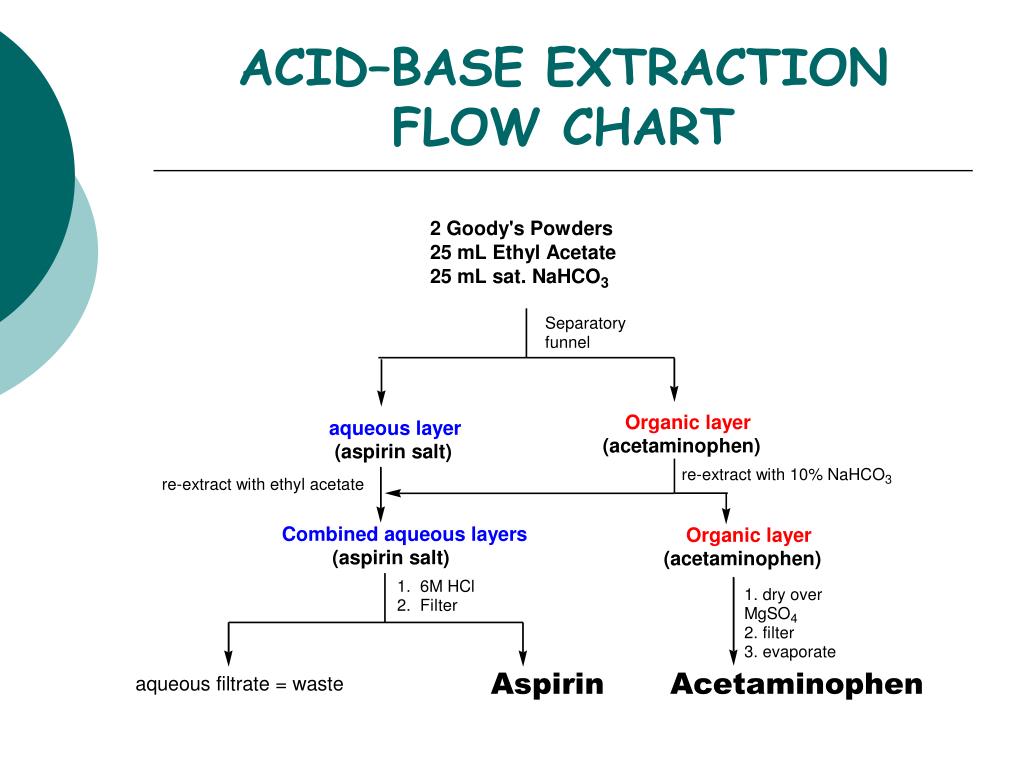
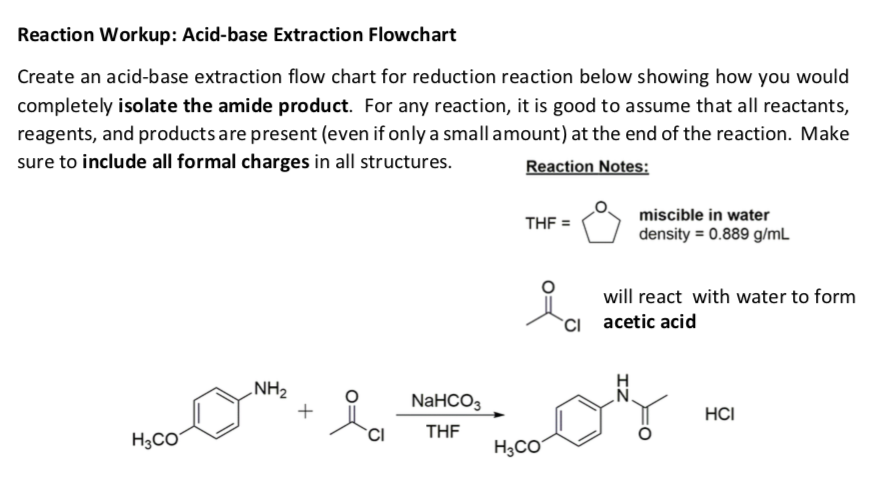
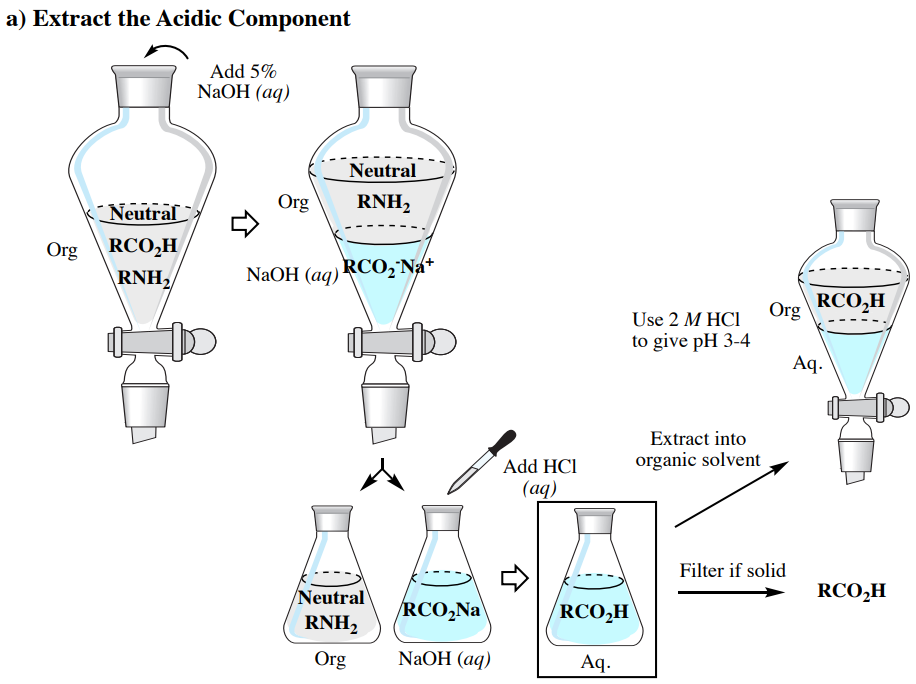
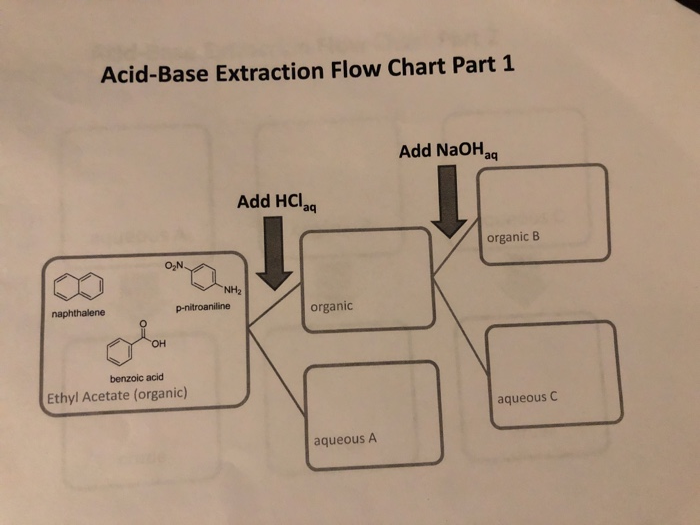
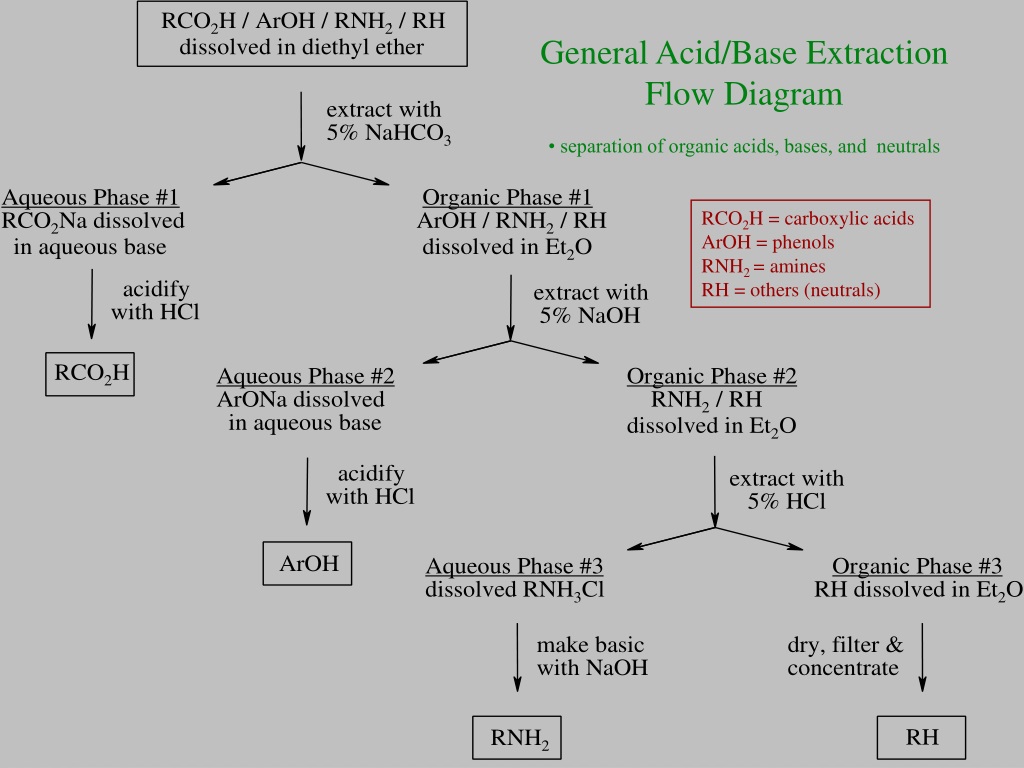
Acid Base Extraction Flow Chart - Web flow chart of acid base extraction. Top organic layer and bottom aqueous layer). Let’s consider a compound x that is dissolved in water. Web the flow chart on the next page outlines a general procedure for separating acidic, basic and neutral organic compounds using the principles of the solubility switch. Phenol will react with a strong base such as sodium. Web acid/base extraction flow chart. 100% (1 rating) here’s how to approach this question. 20 ml of cold 10% hydroxide solution is then added to this organic layer into the separatory funnel. Perform a single extraction using approximately \(25 \: The compounds are an amine, a carboxylic acid and a neutral compound, and the separation depends upon the following properties. Web flow chart of acid base extraction. Submitted 11 july 2007 introduction: Return the aqueous layer to the separatory funnel. Here’s the best way to solve it. Identify the three initial components and determine their solubility in aqueous acid or base to start the separation process by extraction. 100% (1 rating) here’s how to approach this question. 20 ml of 10% bicarbonate is added to the solution in a separatory funnel where the aqueous layer is then removed from the organic layer. 20 ml of cold 10% hydroxide solution is then added to this organic layer into the separatory funnel. Extraction, along with recrystallization and distillation, is one. The mixture is dissolved in 30 ml of diethyl ether. [study aids] combined acidic extract/combined basic extract [study aids] Compound x is water soluble, but is more soluble in diethyl ether. Let’s consider a compound x that is dissolved in water. Submitted 11 july 2007 introduction: Here’s the best way to solve it. Phenol will react with a strong base such as sodium. Web outline a flow chart describing the separation of the mixture and the isolation of each compound. The compounds are an amine, a carboxylic acid and a neutral compound, and the separation depends upon the following properties. Base (rnh2) neutral (n) aqueous phase. What was your percent recovery for each of the three compounds? The mixture is dissolved in 30 ml of diethyl ether. The compounds are an amine, a carboxylic acid and a neutral compound, and the separation depends upon the following properties. Web acid/base extraction flow chart. Aspirin (acetlysalicylic acid) dissolve mixture in about 30ml dichloromethane in erlenmeyer flask and transfer. Let’s consider a compound x that is dissolved in water. Thus its conjugate acid will be positively charged. Common uses in chemical synthesis. Suppose the solubility of x in water is 2.0 g/100 ml, while its solubility in ether is 10.0 g/100 ml. The mixture is dissolved in 30 ml of diethyl ether. 100% (1 rating) here’s how to approach this question. Here’s the best way to solve it. [study aids] combined acidic extract/combined basic extract [study aids] 20 ml of 10% bicarbonate is added to the solution in a separatory funnel where the aqueous layer is then removed from the organic layer. What was your percent recovery for each of the three. The compounds are an amine, a carboxylic acid and a neutral compound, and the separation depends upon the following properties. 20 ml of 10% bicarbonate is added to the solution in a separatory funnel where the aqueous layer is then removed from the organic layer. Thus its conjugate acid will be positively charged. Acid / base extraction pre lab flow. Thus its conjugate acid will be positively charged. Web the flow chart on the next page outlines a general procedure for separating acidic, basic and neutral organic compounds using the principles of the solubility switch. Common uses in chemical synthesis. Perform a single extraction using approximately \(25 \: Aspirin (acetlysalicylic acid) dissolve mixture in about 30ml dichloromethane in erlenmeyer flask. \text{ml}\) of diethyl ether (an exact amount is not necessary), as described previously, making sure to appropriately label each layer (e.g. Acid / base extraction pre lab flow chart. Web outline a flow chart describing the separation of the mixture and the isolation of each compound. Aspirin (acetlysalicylic acid) dissolve mixture in about 30ml dichloromethane in erlenmeyer flask and transfer. Compound x is water soluble, but is more soluble in diethyl ether. Submitted 11 july 2007 introduction: What was your percent recovery for each of the three compounds? Acid / base extraction pre lab flow chart. Assume equal amounts of the carboxylic acid, amine, and neutral compound were present in the unknown mixture. Identify the three initial components and determine their solubility in aqueous acid or base to start the separation process by extraction. Suppose the solubility of x in water is 2.0 g/100 ml, while its solubility in ether is 10.0 g/100 ml. Web acid/base extraction flow chart. Here’s the best way to solve it. Follow the steps, diagrams and examples of this organic chemistry lab technique. [study aids] combined acidic extract/combined basic extract [study aids] \text{ml}\) of diethyl ether (an exact amount is not necessary), as described previously, making sure to appropriately label each layer (e.g. Web outline a flow chart describing the separation of the mixture and the isolation of each compound. Web b) extract the basic component figure 7.8: The compounds are an amine, a carboxylic acid and a neutral compound, and the separation depends upon the following properties. Extraction, along with recrystallization and distillation, is one of the most important separation and purification techniques in.
Flow Chart Of Acid Base Extraction Flow Chart 6B0

Acid Base Extraction Of Alkaloids As the acid/base method is so
PPT Experiment 6 PowerPoint Presentation, free download ID4272400
[Solved] (10pts) Provide a flow chart detailing the acid/base
Acid Base Extraction Flow Chart

acid base extraction of alkaloids Leah Gill
4.8 AcidBase Extraction Chemistry LibreTexts
JulianneoBurgess
PPT Extraction PowerPoint Presentation, free download ID9347367

AcidBase Extraction II Selecting the pH of the Aqueous Layer YouTube
Return The Aqueous Layer To The Separatory Funnel.
100% (1 Rating) Here’s How To Approach This Question.
Top Organic Layer And Bottom Aqueous Layer).
Perform A Single Extraction Using Approximately \(25 \:
Related Post: